A powerful new ‘tool’ for assembling biomolecules
October 21, 2015
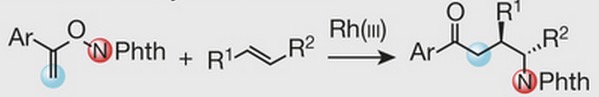
Proposed new simplified chemical reaction for assembling biomolecules in a single chemical reaction (credit: Tiffany Piou & Tomislav Rovis/Nature)
Colorado State University chemists have invented a single chemical reaction that couples two constituent chemicals into a carbon-carbon bond, while simultaneously introducing a nitrogen component. The process promises to replace a multi-step, expensive, and complex process needed when synthesizing new chemicals — for drug creation and testing, for example.
The researchers were able to control this reaction to make the nitrogen atoms go exactly where they want them to, making for precision chemistry that they believe could revolutionize pharmaceutical and biomaterials manufacturing.
The achievement is detailed in the journal Nature, published today (Oct. 21).
Achieving a critical carbon-nitrogen bond
The researchers explain in a statement that “almost every significant carbon-based biomolecule contains a nitrogen compound, or amine. Achieving this carbon-nitrogen bond in the lab, though, is tricky business. Drug companies know it well…. They must first create the carbon-carbon bonds, and then introduce the nitrogen to make a molecule that will do something useful.”
Ball-and-stick model of the ethylene (ethene) molecule, C2H4, the simplest alkene (credit: Benjah-bmm27 CC)
The chemists’ starting materials were simply oil refinery byproducts called olefins, or alkenes. They mixed in a specially engineered reagant, then used a complex based on the precious metal rhodium to reliably and specifically trigger the elusive carbon-nitrogen bonds.
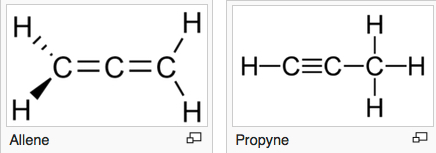
Allene (left) and propyne (right) are examples of isomers containing different bond types (double and triple carbon bonds in this case) — with different functionalities. (credit: Wikipedia)
The innovation also controls molecular isomers (an isomer is a molecule with the same chemical formula as another molecule, but with a different chemical structure). Some isomers are mirror images, like right and left gloves, and although they’re chemically identical, their functionalities are strikingly different. Being able to select for a single isomer is critical to safety and efficacy — so much so that the FDA mandates that only single-isomer drugs be marketed for human use.
Take thalidomide, infamous for causing severe birth defects when taken by pregnant women in the 1950s. Chemically, thalidomide comes in two mirror-image isomeric forms. One caused the defects, one didn’t.
“For this reason, spatial display of groups in molecules is incredibly important,” said organic chemist Tomislav Rovis, professor of chemistry in the College of Natural Sciences at CSU. Rovis led the research with postdoctoral researcher Tiffany Piou, who designed all the chemical building blocks and ran the experiments.
“Tiffany’s finding gives us a leg up to do this in a carboamination reaction, by making the carbon carbon bond, and delivering the nitrogen selectively,” Rovis said.
The researchers hope their approach, which they liken to a tool in a toolbox, can be polished, perfected and used widely to make organic chemistry easier, and applied to many different fields.
Abstract of Rhodium-catalysed syn-carboamination of alkenes via a transient directing group
Alkenes are the most ubiquitous prochiral functional groups—those that can be converted from achiral to chiral in a single step—that are accessible to synthetic chemists. For this reason, difunctionalization reactions of alkenes (whereby two functional groups are added to the same double bond) are particularly important, as they can be used to produce highly complex molecular architectures1, 2. Stereoselective oxidation reactions, including dihydroxylation, aminohydroxylation and halogenation3, 4, 5, 6, are well established methods for functionalizing alkenes. However, the intermolecular incorporation of both carbon- and nitrogen-based functionalities stereoselectively across an alkene has not been reported. Here we describe the rhodium-catalysed carboamination of alkenes at the same (syn) face of a double bond, initiated by a carbon–hydrogen activation event that uses enoxyphthalimides as the source of both the carbon and the nitrogen functionalities. The reaction methodology allows for the intermolecular, stereospecific formation of one carbon–carbon and one carbon–nitrogen bond across an alkene, which is, to our knowledge, unprecedented. The reaction design involves the in situ generation of a bidentate directing group and the use of a new cyclopentadienyl ligand to control the reactivity of rhodium. The results provide a new way of synthesizing functionalized alkenes, and should lead to the convergent and stereoselective assembly of amine-containing acyclic molecules.