Measuring the motion patterns of bacteria in real time
September 18, 2014
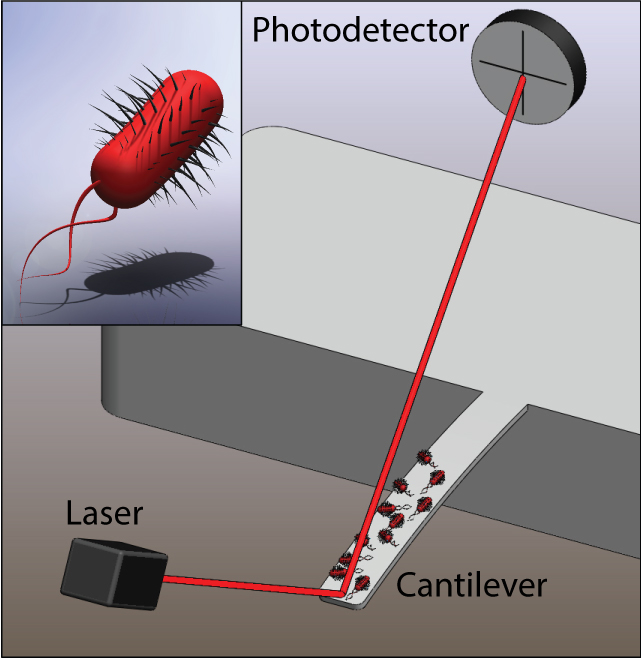
Illustration of a microcantilever sensor with E. coli bacteria attached and a close-up illustration of a single bacterium (inset). The motion of the bacteria couple to the cantilever and the cantilever motion is detected from laser-beam deflection. (Credit: L. Li and C. Lissandrello/Boston University)
Researchers at Boston University and Stanford University School of Medicine have developed a clever new way to study the motion patterns of bacteria in real time — a potential new screen for antibiotics and cancer drugs.
The researchers chemically attached colonies of Escherichia coli bacteria to a microcantilever — a microscopic beam anchored at one end, with the other end movable, and they aimed a laser beam at the cantilever.
Microscopic motions of the bacteria then caused a laser beam deflection that they could use to monitor the colony’s reactions to various stimuli in real time.
“When they die, they stop moving, so it’s a good way to measure the effectiveness of an antibiotic,” said Kamil Ekinci, an associate professor at Boston University. “You know more or less immediately that they’re dead.”
He and fellow researchers described their work in the Sept. 16 issue of the journal Applied Physics Letters.
Instant vs. up to a day with the traditional Petri dish
The traditional method of assessing a bacteria’s antibiotic susceptibility involves culturing bacteria on agar plates infused with antibiotics and waiting up to a day to produce results.
“Here in this system — down to a couple hundred of bacteria — we’re able to see their responses to external stimuli such as drugs,” said Utkan Demirci, an associate professor at Stanford University School of Medicine. “This also potentially applies to other types of cells, such as drug resistance in cancer.”
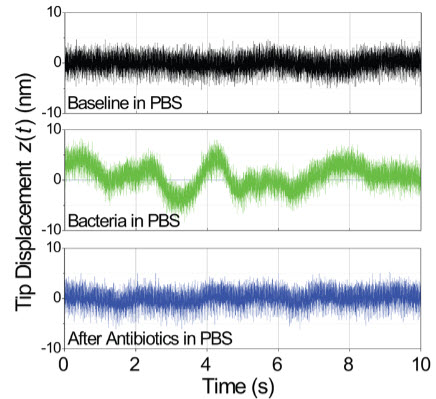
Time domain measurement of microcantilever fluctuations. The top (black) trace is the baseline cantilever fluctuations with no bacteria present. The middle (green) trace is the same measurement after bacteria have adhered to the cantilever surface, indicating in this case about 100 bacteria on the microcantilever. The bottom (blue) trace is after the bacteria are killed in an antibiotic solution. (Credit: C. Lissandrello et al./Applied Physics Letters)
In addition to the long-term goal of creating smaller, portable sensors, future work includes identifying the precise structural sources of vibrations to develop a quantitative physical model of the noise* and better understand the bacterial communication pathways.
The researchers also hope to eventually develop a disposable microfluidic chip, Demirci said. “It could allow us to address some interesting biological questions in the antibiotic resistance and evolution space.”
* Ekinci and Demirci found that when the amplitude of the bacteria’s random movements was plotted against their frequency, a distinct, familiar pattern began to emerge. “We saw that the fluctuations were focused at certain frequencies,” Ekinci said, similar to “pink noise” or “1/f-type noise” (pink noise is similar to white noise).
Pink noise is a recurring pattern in which the power spectral density of a signal is inversely proportional to its frequency. This occurs within a wide variety of systems, including biological processes such as the random firing of neuron channels and the electrocardiogram of a heart’s rhythms, as well as in mechanical processes such as background noise in electronic devices and pitch progression in classical music.
“We think that there are several different time scales in the motion of these bacteria, and when you look at them collectively, you see 1/f-type behavior,” Ekinci said.
Abstract of Applied Physics Letters paper
Nanomechanical motion of bacteria adhered to a chemically functionalized silicon surface is studied by means of a microcantilever. A non-specific binding agent is used to attach Escherichia coli (E. coli) to the surface of a silicon microcantilever. The microcantilever is kept in a liquid medium, and its nanomechanical fluctuations are monitored using an optical displacement transducer. The motion of the bacteria couples efficiently to the microcantilever well below its resonance frequency, causing a measurable increase in the microcantilever fluctuations. In the time domain, the fluctuations exhibit large-amplitude low-frequency oscillations. In corresponding frequency-domain measurements, it is observed that the mechanical energy is focused at low frequencies with a 1/fα -type power law. A basic physical model is used for explaining the observed spectral distribution of the mechanical energy. These results lay the groundwork for understanding the motion of microorganisms adhered to surfaces and for developing micromechanical sensors for bacteria.