Biologists induce flatworms to grow heads and brains of other species
November 25, 2015
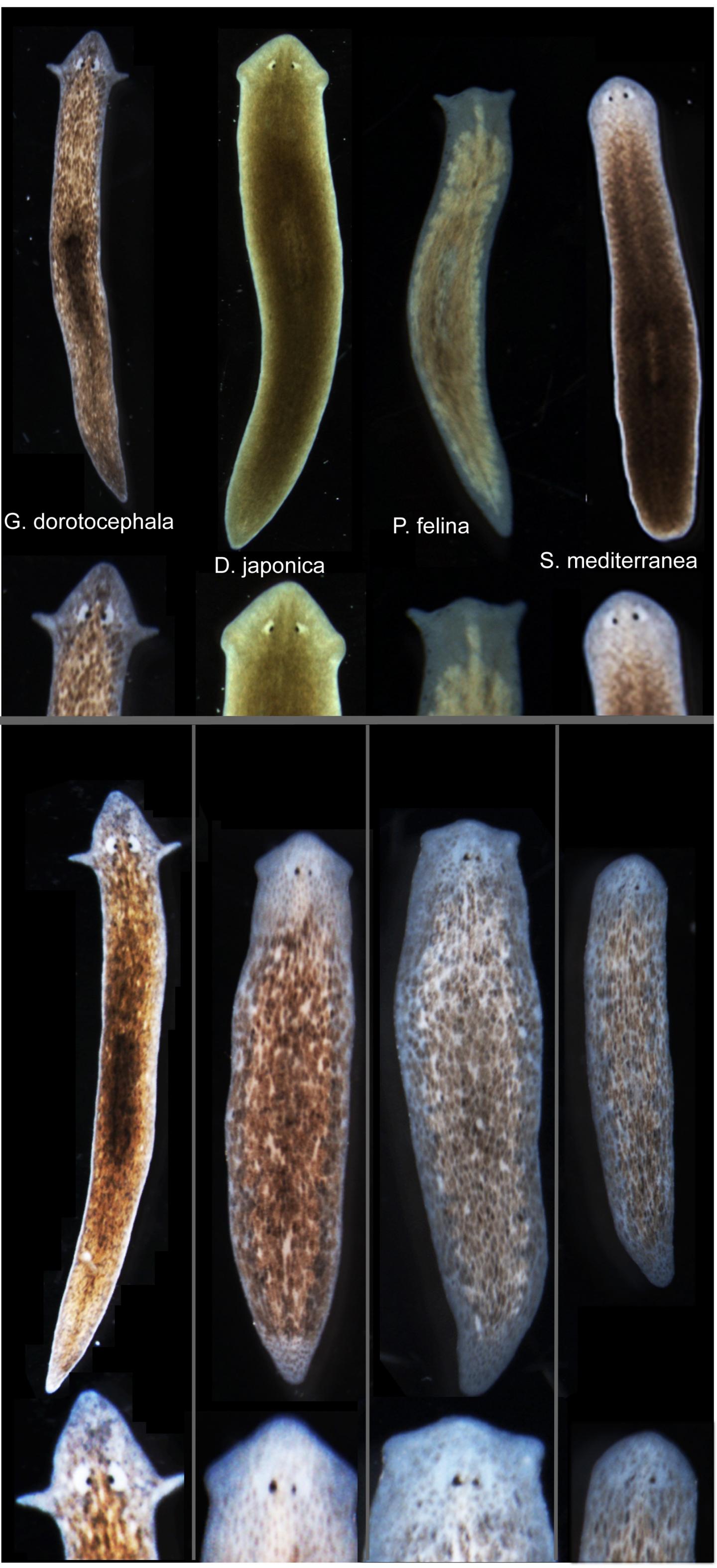
Tufts biologists induced one species of flatworm —- G. dorotocephala, top left — to grow heads and brains characteristic of other species of flatworm, top row, without altering genomic sequence. Examples of the outcomes can be seen in the bottom row of the image. (credit: Center for Regenerative and Developmental Biology, School of Arts and Sciences, Tufts University.)
Tufts University biologists have electrically modified flatworms to grow heads and brains characteristic of another species of flatworm — without altering their genomic sequence. This suggests bioelectrical networks as a new kind of epigenetics (information existing outside of a genomic sequence) to determine large-scale anatomy.
Besides the overall shape of the head, the changes included the shape of the brain and the distribution of the worm’s adult stem cells.
The discovery could help improve understanding of birth defects and regeneration by revealing a new pathway for controlling complex pattern formation similar to how neural networks exploit bioelectric synapses to store and re-write information in the brain.
The findings are detailed in the open-access cover story of the November 2015 edition of the International Journal of Molecular Sciences, appearing online Nov. 24.
“These findings raise significant questions about how genes and bioelectric networks interact to build complex body structures,” said the paper’s senior author Michael Levin, Ph.D., who holds the Vannevar Bush Chair in biology and directs the Center for Regenerative and Developmental Biology in the School of Arts and Sciences at Tufts. Knowing how shape is determined and how to influence it is important because biologists could use that knowledge, for example, to fix birth defects or cause new biological structures to grow after an injury, he explained.
How they did it
The researchers worked with Girardia dorotocephala — free-living planarian flatworms, which have remarkable regenerative capacity. They induced the development of different species-specific head shapes by interrupting gap junctions, which are protein channels that enable cells to communicate with each other by passing electrical signals back and forth.
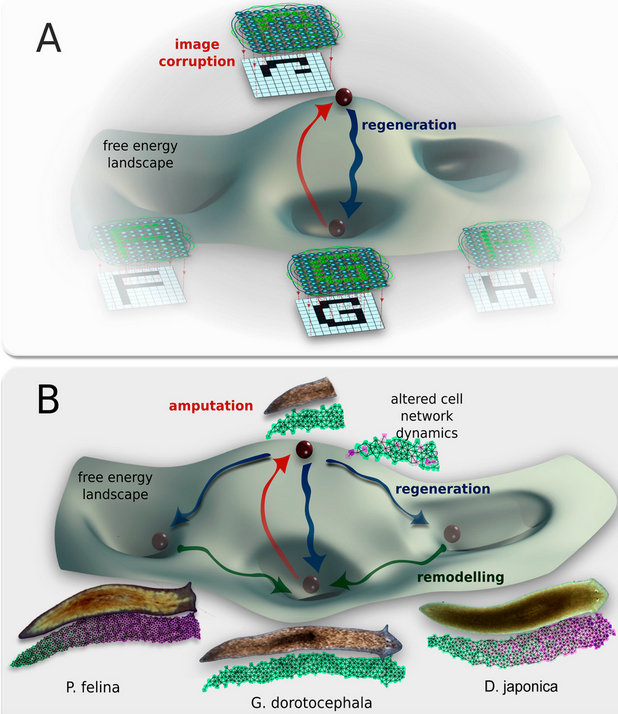
A conceptual model of shape change driven by physiological network dynamics. Planaria regeneration (B) parallels classical neural network behavior (A); both can be described in terms of free energy landscapes with multiple attractor states. (credit: Maya Emmons-Bell et al./Int. J. Mol. Sci.)
The ease with which a particular shape could be coaxed from a G. dorotocephala worm was proportional to the proximity of the target worm on the evolutionary timeline. The closer the two species were related, the easier it was to effect the change. This observation strengthens the connection to evolutionary history, suggesting that modulation of physiological circuits may be one more tool exploited by evolution to alter animal body plans.
However, this shape change was only temporary. Weeks after the planaria completed regeneration to the other species’ head shapes, the worms once again began remodeling and re-acquired their original head morphology. Additional research is needed to determine how this occurs. The authors also presented a computational model that explains how changes in cell-to-cell communication can give rise to the diverse shape types.
The interdisciplinary research involved U.S.- and Canada-based biologists and European mathematicians.
Abstract of Gap Junctional Blockade Stochastically Induces Different Species-Specific Head Anatomies in Genetically Wild-Type Girardia dorotocephala Flatworms
The shape of an animal body plan is constructed from protein components encoded by the genome. However, bioelectric networks composed of many cell types have their own intrinsic dynamics, and can drive distinct morphological outcomes during embryogenesis and regeneration. Planarian flatworms are a popular system for exploring body plan patterning due to their regenerative capacity, but despite considerable molecular information regarding stem cell differentiation and basic axial patterning, very little is known about how distinct head shapes are produced. Here, we show that after decapitation in G. dorotocephala, a transient perturbation of physiological connectivity among cells (using the gap junction blocker octanol) can result in regenerated heads with quite different shapes, stochastically matching other known species of planaria (S. mediterranea, D. japonica, and P. felina). We use morphometric analysis to quantify the ability of physiological network perturbations to induce different species-specific head shapes from the same genome. Moreover, we present a computational agent-based model of cell and physical dynamics during regeneration that quantitatively reproduces the observed shape changes. Morphological alterations induced in a genomically wild-type G. dorotocephala during regeneration include not only the shape of the head but also the morphology of the brain, the characteristic distribution of adult stem cells (neoblasts), and the bioelectric gradients of resting potential within the anterior tissues. Interestingly, the shape change is not permanent; after regeneration is complete, intact animals remodel back to G. dorotocephala-appropriate head shape within several weeks in a secondary phase of remodeling following initial complete regeneration. We present a conceptual model to guide future work to delineate the molecular mechanisms by which bioelectric networks stochastically select among a small set of discrete head morphologies. Taken together, these data and analyses shed light on important physiological modifiers of morphological information in dictating species-specific shape, and reveal them to be a novel instructive input into head patterning in regenerating planaria.