Blood-brain-barrier disruption with high-frequency pulsed electric fields
August 14, 2014
A cortical microvessel stained for blood-brain-barrier protein ZO-1 (credit: Nathan S. Ivey/Wikimedia Commons/)
A team of researchers from Virginia Tech and Wake Forest University School of Biomedical Engineering and Sciences have developed a new technique for using pulsed electric energy to open the blood-brain-barrier (BBB) for treating brain cancer and neurological disorders.
Their Vascular Enabled Integrated Nanosecond pulse (VEIN pulse) procedure consists of inserting minimally invasive needle electrodes into the diseased tissue and applying multiple bursts of 850-nanosecond pulsed electric energy with alternating polarity.
The researchers think the bursts disrupt tight junction proteins responsible for maintaining the integrity of the BBB, but without causing damage to the surrounding tissue.
This technique will be described in the upcoming issue of the journal TECHNOLOGY.
Treating brain diseases
For the treatment of brain cancer, “VEIN pulses could be applied at the same time as biopsy or through the same track as the biopsy probe in order to mitigate damage to the healthy tissue by limiting the number of needle insertions,” says Rafael V. Davalos, Ph.D, director of the Bioelectromechanical Systems Laboratory at Virginia Tech.
The BBB is a network of tight junctions that normally acts to protect the brain from foreign substances by preventing them from leaking from blood vessels into neural structures. But that also limits the effectiveness of drugs to treat brain disease. Temporarily opening the BBB is a way to ensure that drugs can still be effective.
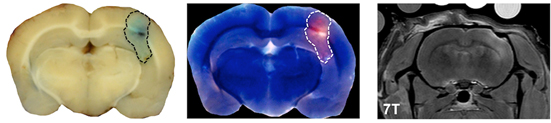
Pathologic and MRI evidence of blood-brain-barrier (BBB) disruption induced by the VEIN (Vascular Enabled Integrated Nanosecond) pulse generation system. Two minimally invasive needle electrodes with 1 mm active length were spaced 4.0 mm apart and inserted into the right cerebral hemisphere 1.5 mm beneath the surface of the dura. A burst of 200, 500 ns duration square pulses of alternating polarity with a voltage-to-distance ratio of 250 V/cm were applied through the electrodes. In the case shown above, bursts were repeated once per second for 10 min. The extent of BBB disruption is shown by the dotted line surrounding Evans blue-albumin complex uptake on the gross brain slice preparation (left) and the corresponding fluorescent image (middle). Additionally, areas of BBB disruption appear as hyperintense (white) on the T1-weighted MRI exam, due to the uptake of a gadolinium-Evans blue tracer. Scale bar represents 5 mm. (Credit: John H. Rossmeisl Jr., Neurology and Neurosurgery, Virginia-Maryland Regional College of Veterinary Medicine and Virginia Tech-Wake Forest University School of Biomedical Engineering and Sciences).
The research also shows that VEIN pulses can be applied without causing muscle contractions, which may dislodge the electrodes and require the use of a neuroblocker and general anesthesia. According to Christopher B. Arena, Ph.D., co-lead author on the paper, “the fact that the pulses alternate in polarity helps to avoid unwanted, electrically induced movement. Therefore, it could be possible to perform this procedure without using a neuroblocker and with patients under conscious sedation. This is similar to how deep brain stimulation is implemented clinically to treat Parkinson’s disease.”
The team now plans to translate the technology into clinical applications through a university spin-out company, VoltMed, Inc.
Researchers at Virginia-Maryland Regional College of Veterinary Medicine where also involved in the study.
This research was supported in part by grants from the National Science Foundation, the Golfers Against Cancer, and the Center for Biomolecular Imaging in the Wake Forest School of Medicine.
Abstract of Technology paper