Chinese team genetically modifies human embryo, using CRISPR gene-editing technique
April 11, 2016
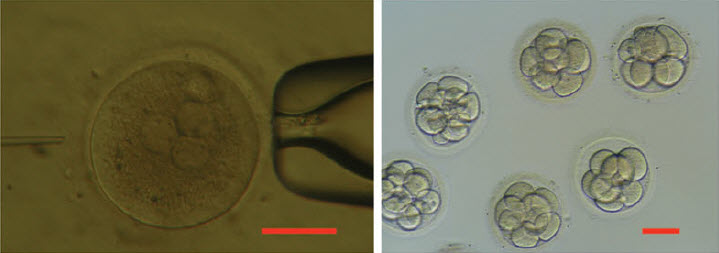
Photos showing injection of a human 3PN zygote (left) and development to 8 to 16-cell stage in vitro (right). Scale bar = 100 μm. (credit: Xiangjin Kang et al./Journal of Assisted Reproduction and Genetics)
Chinese researchers have genetically modified a human embryo using CRISPR/Cas9, the gene editing technique, using embryos that carried an extra set of chromosomes (so they were not viable) — hoping to learn more about the possibility of producing human babies that would be immune to HIV.
The Chinese team reports in the Journal of Assisted Reproduction and Genetics that they obtained 213 fertilized eggs from a fertility clinic, which had been deemed unsuitable for in vitro therapy.* They used the eggs to study a mutation that causes damage to an immune cell gene called CCR5 (this type of cell, when damaged naturally, has been found to lead to HIV resistance).
Aside from a previous study in China last year that involved editing human embryo genes (see Researchers in China have created genetically modified human embryos), most of the world has decided to hold off doing this controversial research. “We believe that any attempt to generate genetically modified humans through the modification of early embryos needs to be strictly prohibited until we can resolve both ethical and scientific issues,” the researchers say in their paper.
Failed experiment
The team reports that just 4 out of 26 of the embryos that were edited were modified successfully — some still contained genes that had not been modified, and others had resulted in unexpected gene mutations.
“This paper doesn’t look like it offers much more than anecdotal evidence that it works in human embryos, which we already knew,” George Daley, a stem-cell biologist at Children’s Hospital Boston, told Nature. “It’s certainly a long way from realizing the intended potential” — a human embryo with all its copies of CCR5 inactivated.
“It just emphasizes that there are still a lot of technical difficulties to doing precision editing in human embryo cells,” Xiao-Jiang Li, a neuroscientist at Emory University, also said in the Nature article . He thinks that researchers should work out these kinks in non-human primates, for example, before continuing to modify the genomes of human embryos using techniques such as CRISPR.
* The women who had donated the eggs all gave permission for the embryos to be used for genetic research, on condition that the embryos would not be allowed to mature into a human being (all of the embryos were destroyed after three days).
Abstract of Introducing precise genetic modifications into human 3PN embryos by CRISPR/Cas-mediated genome editing
Purpose
As a powerful technology for genome engineering, the CRISPR/Cas system has been successfully applied to modify the genomes of various species. The purpose of this study was to evaluate the technology and establish principles for the introduction of precise genetic modifications in early human embryos.
Methods
3PN zygotes were injected with Cas9 messenger RNA (mRNA) (100 ng/μl) and guide RNA (gRNA) (50 ng/μl). For oligo-injections, donor oligo-1 (99 bp) or oligo-2 (99 bp) (100 ng/μl) or dsDonor (1 kb) was mixed with Cas9 mRNA (100 ng/μl) and gRNA (50 ng/μl) and injected into the embryos.
Results
By co-injecting Cas9 mRNA, gRNAs, and donor DNA, we successfully introduced the naturally occurring CCR5Δ32 allele into early human 3PN embryos. In the embryos containing the engineeredCCR5Δ32 allele, however, the other alleles at the same locus could not be fully controlled because they either remained wild type or contained indel mutations.
Conclusions
This work has implications for the development of therapeutic treatments of genetic disorders, and it demonstrates that significant technical issues remain to be addressed. We advocate preventing any application of genome editing on the human germline until after a rigorous and thorough evaluation and discussion are undertaken by the global research and ethics communities.