Embryonic stem cells appear safe, may help eye disease
January 24, 2012 | Source: Science Now
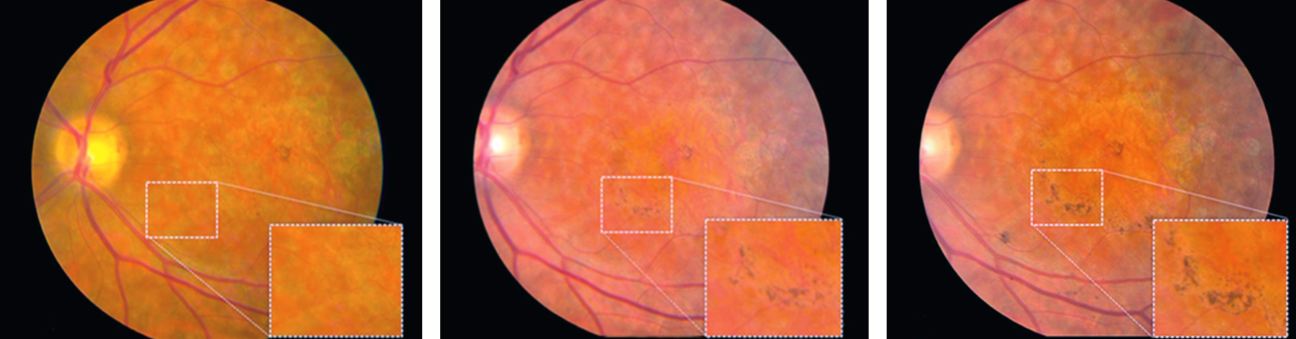
Images of the hESC-RPE transplantation site in the patient with Stargardt’s macular dystrophy. Color fundus photographs of the patient’s left macula preoperatively and postoperatively. (credit: Steven D Schwartz et al.)
In the first published results from a clinical trial using human embryonic stem cells, two legally blind patients who received an injection of hESC-derived cells in one eye have experienced no harmful side effects and appear to have slightly better vision. Although the result is preliminary, it is an important milestone for the struggling hESC field.
In the only ongoing clinical trials involving hESCs, the biotechnology company Advanced Cell Technology (ACT) of Marlborough, Massachusetts, derived a type of cell called retinal pigment epithelium (RPE), which sustains the retina’s light-sensing photoreceptors. Collaborators at the University of California, Los Angeles, then used these cells to treat an elderly woman with macular degeneration and a woman with macular dystrophy.
Four months after receiving the treatment, the patients have not developed any tumors or abnormal growths that have sometimes been seen in animals receiving hESC-derived cells, the team reports online in The Lancet. The patients, who received immune-suppressing drugs for 12 weeks to prevent their bodies from attacking the cells, also have not shown inflammation or other signs of rejecting the cells.
“It’s a long time coming. It’s rewarding to finally see years and years of research translated to the bedside,” says Robert Lanza, CEO of ACT.
Ref.: Steven D Schwartz et al., Embryonic stem cell trials for macular degeneration:
a preliminary report, The Lancet, 2012 [DOI: 10.1016/S0140-6736(12)60028-2]