Future of drug delivery seen in a crystal ball
February 3, 2016
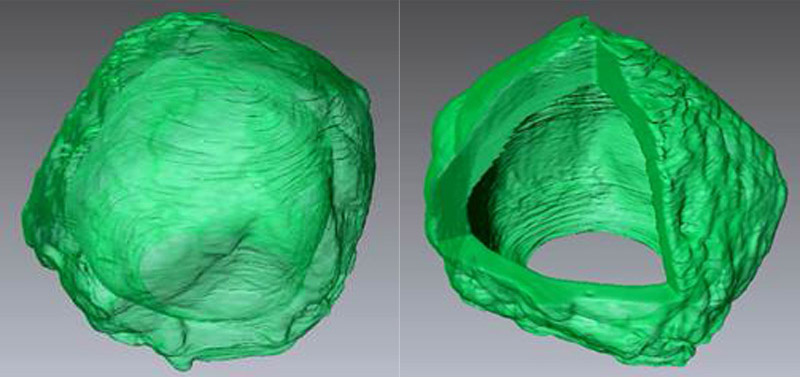
Researchers have developed a way to grow crystals in a spherical shape — a possible future drug-delivery platform. (credit: Drexel University)
A Drexel University materials scientist has discovered a way to encapsulate medication to deliver it more effectively inside the body.
Until now, crystals have grown in rigid, structured formations (like the snowflake) — with a web of straight lines connecting to making a grid that grows into the crystalline flake.*
But the formation of a crystal is affected by the environment in which it forms. And Christopher Li, PhD, a professor in the College of Engineering and head of the Soft Materials Lab in the Department of Materials Science & Engineering, uses this workaround to engineer hollow crystal spheres. He recently reported his finding in Nature Communications (open access).
Introducing …. the “crystalsome”
Li was able to overcome crystal’s edge-forming tendencies by creating a tiny bubble of oil to encase water molecules. When the surfactant bubble was cooled to the appropriate temperature, the molecules inside began to crystalize. But rather than forming an angular web of connections, the molecules, instead, lined up along the interior of the oil bubble — crystallizing in a hollow, spherical shape.
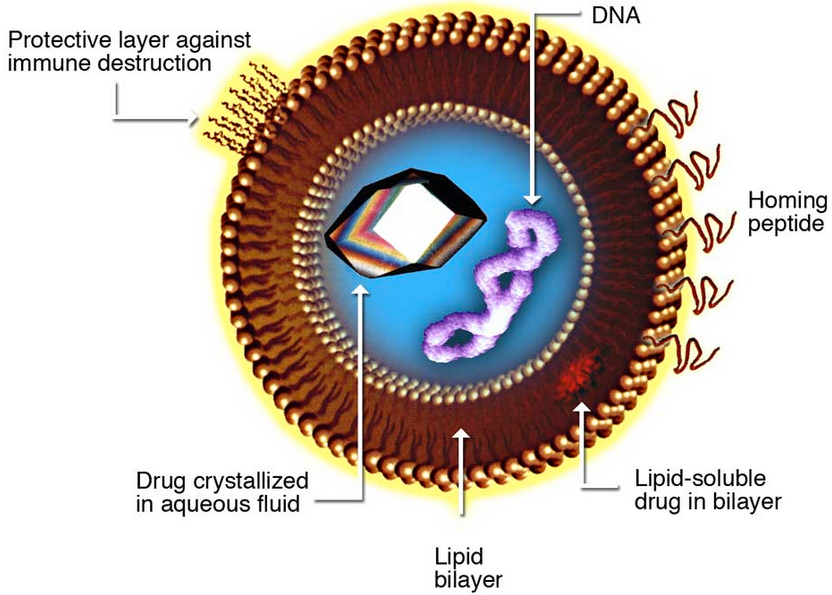
Liposomes, composite structures made of phospholipids, can carry drugs to target tissues (Kosi Gramatikoff/Wikimedia Commons)
Early tests indicate that Li’s “crystalsome” (named for their similarity to liposomes — tiny bubbles with the same membrane as cells that are being explored for use as biological packages for delivering drug treatments) is a few hundred times stronger than liposomes, making them a sturdier option for medicine encapsulation.
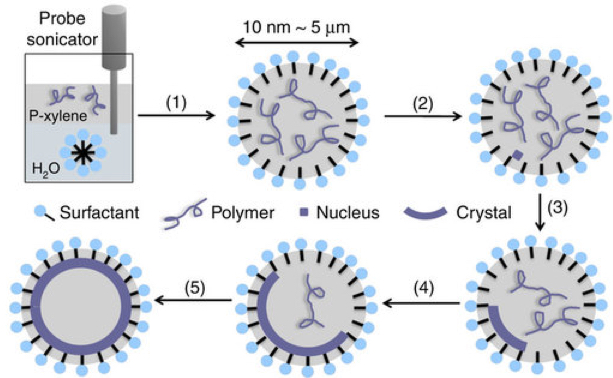
Crystalsome fabriation process: 1) Emulsification; (2) quench to the crystallization temperature; and (3–5) different stages of crystal growth (credit: Wenda Wang et al./Nature Communications)
With funding from the National Science Foundation, Li’s team is now exploring ways to control the shape and strength of the spheres by making them out of various different molecules.
* Crystals form this way because their molecules are predisposed to align themselves in a way that links them via the strongest electrochemical bond available. If molecules are floating freely, as they are in a water vapor for example, they are able to follow this default course to connect with other molecules and, eventually, form a crystal — an ice crystal, or snowflake, in the case of water molecules.
Abstract of Highly robust crystalsome via directed polymer crystallization at curved liquid/liquid interface
Lipids and amphiphilic block copolymers spontaneously self-assemble in water to form a plethora of micelles and vesicles. They are typically fluidic in nature and often mechanically weak for applications such as drug delivery and gene therapeutics. Mechanical properties of polymeric materials could be improved by forming crystalline structures. However, most of the self-assembled micelles and vesicles have curved surfaces and precisely tuning crystallization within a nanoscale curved space is challenging, as the curved geometry is incommensurate with crystals having three-dimensional translational symmetry. Herein, we report using a miniemulsion crystallization method to grow nanosized, polymer single-crystal-like capsules. We coin the name crystalsome to describe this unique structure, because they are formed by polymer lamellar crystals and their structure mimics liposomes and polymersomes. Using poly(L-lactic acid) (PLLA) as the model polymer, we show that curved water/p-xylene interface formed by the miniemulsion process can guide the growth of PLLA single crystals. Crystalsomes with the size ranging from ~148 nm to over 1 μm have been formed. Atomic force microscopy measurement demonstrate a two to three orders of magnitude increase in bending modulus compared with conventional polymersomes. We envisage that this novel structure could shed light on investigating spherical crystallography and drug delivery.