Gold nanotubes image and destroy cancer cells in three ways
February 17, 2015
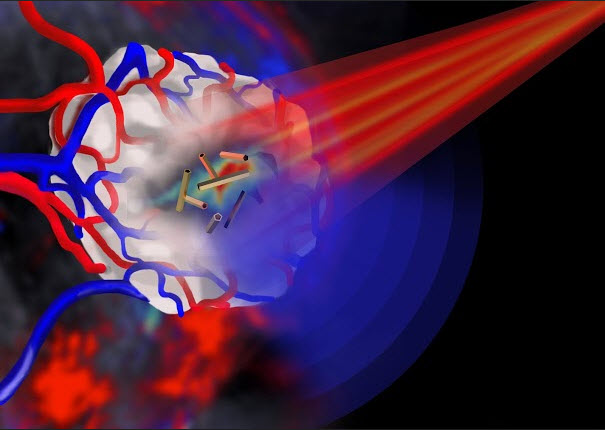
Pulsed near infrared light (shown in red) is shone onto a tumor (shown in white) that is encased in blood vessels. The tumor is imaged by multispectral optoacoustic tomography via the ultrasound emission (shown in blue) from the gold nanotubes. (credit: Jing Claussen/iThera Medical, Germany)
Leeds scientists have shown that gold nanotubes can fight cancer in three ways: as internal nanoprobes for high-resolution photoacoustic imaging, as drug delivery vehicles, and as agents for destroying cancer cells.
The study, published in the journal Advanced Functional Materials, details the first successful demonstration of the biomedical use of gold nanotubes in a mouse model of human cancer — an alternative to existing chemotherapy and radiotherapy methods, which have serious side effects.
“To the best of our knowledge, this is the first [combination] in vitro [lab] and in vivo [live in animals] study of gold nanotubes,” the researchers say.
According to study lead author Sunjie Ye, who is based in the School of Physics and Astronomy and the Leeds Institute for Biomedical and Clinical Sciences at the University of Leeds, “high recurrence rates of tumors after surgical removal remain a formidable challenge in cancer therapy. Gold nanotubes have the potential to enhance the efficacy of these conventional treatments by integrating diagnosis and therapy in one single system.”
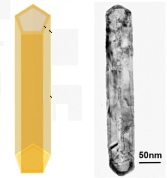
Gold nanotube schematic showing hollow interior (left) and transmission electron microscope image (right) (credit: Jeremy Freear/Advanced Functional Materials)
The researchers injected the gold nanotubes intravenously. They controlled the length of the nanotubes for the right dimensions to absorb near-infrared light (which penetrates tissue well) from a pulsed infrared laser beam.
By adjusting the brightness of the laser pulse, the researchers were able to control whether the gold nanotubes were in imaging mode or cancer-destruction mode.
For imaging, after absorbing energy from the laser pulse, the gold nanotubes generated ultrasound for multispectral optoacoustic tomography (MSOT), used to detect the gold nanotubes.
For cancer destruction, there were two options:
- Use a stronger laser beam to rapidly raise the temperature in the vicinity of the nanotubes so that the temperature was high enough to destroy cancer cells.
- Load the central hollow core of the nanotubes with a therapeutic payload.
The gold nanotubes were coated with protective sodium polystyrenesulfonate (PSS) and were excreted from the body, and therefore are unlikely to cause problems in terms of toxicity, an important consideration when developing nanoparticles for clinical use, the researchers say.
Abstract of Engineering gold nanotubes with controlled length and near-infrared absorption for theranostic applications
Important aspects in engineering gold nanoparticles for theranostic applications include the control of size, optical properties, cytotoxicity, biodistribution, and clearance. In this study, gold nanotubes with controlled length and tunable absorption in the near-infrared (NIR) region have been exploited for applications as photothermal conversion agents and in vivo photoacoustic imaging contrast agents. A length-controlled synthesis has been developed to fabricate gold nanotubes (NTs) with well-defined shape (i.e., inner void and open ends), high crystallinity, and tunable NIR surface plasmon resonance. A coating of poly(sodium 4-styrenesulfonate) (PSS) endows the nanotubes with colloidal stability and low cytotoxicity. The PSS-coated Au NTs have the following characteristics: i) cellular uptake by colorectal cancer cells and macrophage cells, ii) photothermal ablation of cancer cells using single wavelength pulse laser irradiation, iii) excellent in vivo photoacoustic signal generation capability and accumulation at the tumor site, iv) hepatobiliary clearance within 72 h postintravenous injection. These results demonstrate that these PSS-coated Au NTs have the ideal attributes to develop their potential as effective and safe in vivo imaging nanoprobes, photothermal conversion agents, and drug delivery vehicles. To the best of knowledge, this is the first in vitro and in vivo study of gold nanotubes.