Graphene is ideal substrate for brain electrodes, researchers find
February 1, 2016
This illustration portrays neurons interfaced with a sheet of graphene molecules in the background (credit: Graphene Flagship)
An international study headed by the European Graphene Flagship research consortium has found that graphene is a promising material for use in electrodes that interface with neurons, based on its excellent conductivity, flexibility for molding into complex shapes, biocompatibility, and stability within the body.
The graphene-based substrates they studied* promise to overcome problems with “glial scar” tissue formation (caused by electrode-based brain trauma and long-term inflammation). To avoid that, current electrodes based on tungsten or silicon use a protective coating on electrodes, which reduces charge transfer. Current electrodes are also rigid (resulting in tissue detachment and preventing neurons from moving) and generate electrical noise, with partial or complete loss of signal over time, the researchers note in a paper published recently in the journal ACS Nano.
Electrodes are used as neural biosensors and for prosthetic applications — such as deep-brain intracranial electrodes used to control motor disorders (mainly epilepsy or Parkinson’s) and for brain-computer interfaces (BCIs), used to recover sensory functions or control robotic arms for paralyzed patients. These applications require an interface with long-term, minimal interference.
Interfacing graphene to neurons directly
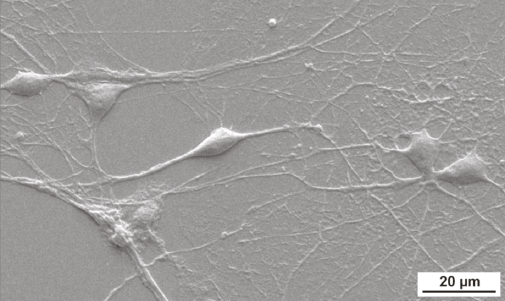
Scanning electron microscope image of rat hippocampal neurons grown in the lab on a graphene-based substrate, showing normal morphology characterized by well-defined round neural soma, extended neurite arborization (branching), and cell density similar to control substrates (credit: A. Fabbro et al./ACS Nano)
“For the first time, we interfaced graphene to neurons directly, without any peptide-coating,” explained lead neuroscientist Prof. Laura Ballerini of the International School for Advanced Studies (SISSA/ISAS) and the University of Trieste.
Using electron microscopy and immunofluorescence, the researchers found that the neurons remained healthy, transmitting normal electric impulses and, importantly, no adverse glial reaction, which leads to damaging scar tissue, was seen.
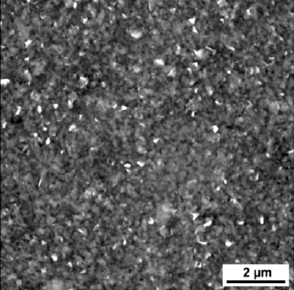
Atomic force microscope (AFM) image of graphene-based substrate created using liquid phase exfoliation (credit: A. Fabbro et al./ACS Nano)
As a next step, Ballerini says the team plans to investigate how different forms of graphene, from multiple layers to monolayers, are able to affect neurons, and “whether tuning the graphene material properties might alter the synapses and neuronal excitability in new and unique ways.”
Prof. Andrea C. Ferrari, Director of the Cambridge Graphene Centre and Chair of the Graphene Flagship Executive Board, said the Flagship will “support biomedical research and development based on graphene technology with a new work package and a significant cash investment from 2016.”
The interdisciplinary collaboration also included the University Castilla-La Mancha and the Cambridge Graphene Centre.
* The study used two methods of creating graphene-based substrates (GBSs). Liquid phase exfoliation (LPE) — peeling off graphene from graphite — can be performed without the potentially hazardous chemical treatments involved in graphene oxide production, is scalable, and operates at room temperature, with high yield. LPE dispersions can also be easily deposited on target substrates by drop-casting, filtration, or printing. Ball milling (BM), with the help of melamine (which forms large hydrogen-bond domains, unlike LPE), can be performed in a solid environment. “Our data indicate that both GBSs are promising for next-generation bioelectronic systems, to be used as brain interfaces,” the paper concludes.