Groundbreaking technology rewarms large-scale animal tissues preserved at low temperatures
March 2, 2017
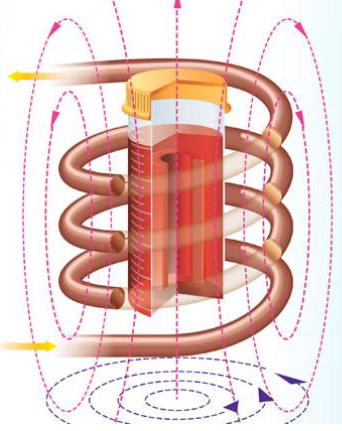
Inductive radio-frequency heating of magnetic nanoparticles embedded in tissue (red material in container) preserved at very low temperatures restored the tissue without damage (credit: Navid Manuchehrabadi et al./Science Translational Medicine)
A research team led by the University of Minnesota has discovered a way to rewarm large-scale animal heart valves and blood vessels preserved at very low (cryogenic) temperatures without damaging the tissue. The discovery could one day lead to saving millions of human lives by creating cryogenic tissue and organ banks of organs and tissues for transplantation.
The research was published March 1 in an open-access paper in Science Translational Medicine.
Long-term preservation methods like vitrification cool biological samples to an ice-free glassy state, using very low temperatures between -160 and -196 degrees Celsius, but tissues larger than 1 milliliter (0.03 fluid ounce) often suffer major damage during the rewarming process, making them unusable for tissues.
In the new research, the researchers were able to restore 50 milliliters (1.7 fluid ounces) of tissue with warming at more than 130°C/minute without damage.
Radiofrequency inductive heating of iron nanoparticles
To achieve that, they developed a revolutionary new method using silica-coated iron-oxide nanoparticles dispersed throughout a cryoprotectant solution around the tissue. The nanoparticles act as tiny heaters around the tissue when they are activated using noninvasive radiofrequency inductive energy, rapidly and uniformly warming the tissue.
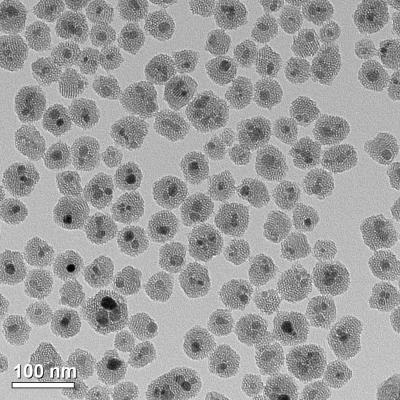
This transmission electron microscopy (TEM) image shows the iron oxide nanoparticles (coated in mesoporous silica) that are used in the tissue warming process. (credit: Haynes research group/University of Minnesota)
The results showed that none of the tissues displayed signs of harm — unlike control samples using vitrification and rewarmed slowly over ice or using convection warming. The researchers were also able to successfully wash away the iron oxide nanoparticles from the sample following the warming.
“This is the first time that anyone has been able to scale up to a larger biological system and demonstrate successful, fast, and uniform warming of hundreds of degrees Celsius per minute of preserved tissue without damaging the tissue,” said University of Minnesota mechanical engineering and biomedical engineering professor John Bischof, the senior author of the study.
Organs next
Bischof said there is a strong possibility they could scale up to even larger systems, like organs. The researchers plan to start with rodent organs (such as rat and rabbit) and then scale up to pig organs and then, hopefully, human organs. The technology might also be applied beyond cryogenics, including delivering lethal pulses of heat to cancer cells.
The researchers’ goal is to eliminate transplant waiting lists. Currently, hearts and lungs donated for transplantation must be discarded because these tissues cannot be kept on ice for longer than a matter of hours, according to the researchers.*
It will be interesting to see if the technology can one day be extended to cryonics.
The research was funded by the National Science Foundation (NSF), National Institutes of Health (NIH), U.S. Army Medical Research and Materiel Command, Minnesota Futures Grant from the University of Minnesota, and the University of Minnesota Carl and Janet Kuhrmeyer Chair in Mechanical Engineering. Researchers at Carnegie Mellon University, Clemson University and Tissue Testing Technologies LLC were also involved in the study.
* “A major limitation of transplantation is the ischemic injury that tissue and organs sustain during the time between recovery from the donor and implantation in the recipient. The maximum tolerable organ preservation for transplantation by hypothermic storage is typically 4 hours for heart and lungs; 8 to 12 hours for liver, intestine, and pancreas; and up to 36 hours for kidney transplants. In many cases, such limits actually prevent viable tissue or organs from reaching recipients. For instance, more than 60% of donor hearts and lungs are not used or transplanted partly because their maximum hypothermic preservation times have been exceeded. Further, if only half of these discarded organs were transplanted, then it has been estimated that wait lists for these organs could be extinguished within 2 to 3 years.” — Navid Manuchehrabadi et al./Science Translational Medicine
Abstract of Improved tissue cryopreservation using inductive heating of magnetic nanoparticles
Vitrification, a kinetic process of liquid solidification into glass, poses many potential benefits for tissue cryopreservation including indefinite storage, banking, and facilitation of tissue matching for transplantation. To date, however, successful rewarming of tissues vitrified in VS55, a cryoprotectant solution, can only be achieved by convective warming of small volumes on the order of 1 ml. Successful rewarming requires both uniform and fast rates to reduce thermal mechanical stress and cracks, and to prevent rewarming phase crystallization. We present a scalable nanowarming technology for 1- to 80-ml samples using radiofrequency-excited mesoporous silica–coated iron oxide nanoparticles in VS55. Advanced imaging including sweep imaging with Fourier transform and microcomputed tomography was used to verify loading and unloading of VS55 and nanoparticles and successful vitrification of porcine arteries. Nanowarming was then used to demonstrate uniform and rapid rewarming at >130°C/min in both physical (1 to 80 ml) and biological systems including human dermal fibroblast cells, porcine arteries and porcine aortic heart valve leaflet tissues (1 to 50 ml). Nanowarming yielded viability that matched control and/or exceeded gold standard convective warming in 1- to 50-ml systems, and improved viability compared to slow-warmed (crystallized) samples. Last, biomechanical testing displayed no significant biomechanical property changes in blood vessel length or elastic modulus after nanowarming compared to untreated fresh control porcine arteries. In aggregate, these results demonstrate new physical and biological evidence that nanowarming can improve the outcome of vitrified cryogenic storage of tissues in larger sample volumes.