How to create ultra-low-cost solar cells from agricultural waste
February 8, 2012
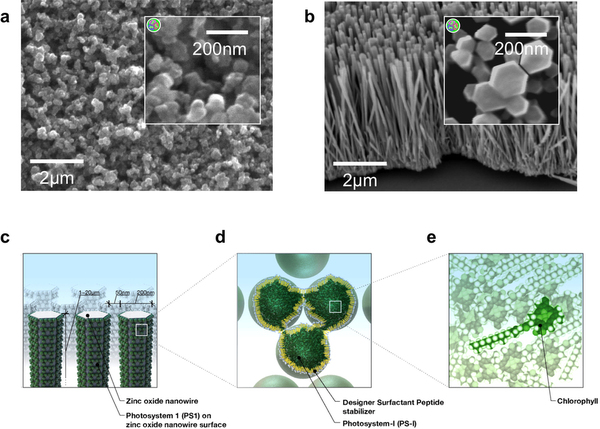
Nanostructured titanium oxide and zinc oxide photoanodes and schematic of an ideal ultra-low cost biophotovoltaic arrangement
Within a few years, people in remote villages in the developing world may be able to make their own solar panels, at low cost, using otherwise worthless agricultural waste as their raw material.
That’s the vision of MIT researcher Andreas Mershin. He says the process has been simplified to the point that virtually any lab could replicate it — including college or even high school science labs — allowing researchers around the world to start exploring the process and making further improvements.
The new system’s efficiency is 10,000 times greater than in the previous version — although in converting just 0.1 percent of sunlight’s energy to electricity, it still needs to improve another tenfold or so to become useful, he says.
Mershin was able to create a tiny forest of zinc oxide (ZnO) nanowires as well as a sponge-like titanium dioxide (TiO2) nanostructure coated with the light-collecting material derived from bacteria. The nanowires not only served as a supporting structure for the material, but also as wires to carry the flow of electrons generated by the molecules down to the supporting layer of material, from which it could be connected to a circuit. “It’s like an electric nanoforest,” he says.
As an bonus, both zinc oxide and titanium dioxide — the main ingredient in many sunscreens — are very good at absorbing ultraviolet light.
Mershin thinks that because he and his colleagues have now lowered the barrier to entry for further work on these materials, progress toward improving their efficiency should be rapid. Ultimately, once the efficiency reaches 1 or 2 percent, he says, that will be good enough to be useful, because the ingredients are so cheap and the processing so simple.
“You can use anything green, even grass clippings” as the raw material, he says — in some cases, waste that people would otherwise pay to have hauled away. The team has proposed a way to achieve this concentration by using inexpensive membranes for filtration. No special laboratory conditions are needed, Mershin says: “It can be very dirty and it still works, because of the way nature has designed it. Nature works in dirty environments — it’s the result of billions of experiments over billions of years.”
Because the system is so cheap and simple, he hopes this will become a “way of getting low-tech electricity to people who have never been thought of as consumers or producers of solar-power technology.” He hopes the instructions for making a solar cell will be simple enough to be reduced to “one sheet of cartoon instructions, with no words.” The only ingredient to be purchased would be chemicals to stabilize the chemicals, which could be packaged inexpensively in a plastic bag.
Essentially, Mershin says, within a few years a villager in a remote, off-grid location could “take that bag, mix it with anything green and paint it on the roof” to start producing power, which could then charge cellphones or lanterns. Today, the most widely used source of lighting in such locations is kerosene lanterns — “the most expensive, most unhealthy” form of lighting there is, he says. “Nighttime illumination is the number one way to get out of poverty,” he adds, because it enables people who work in the fields all day to read at night and get an education.
Ref.: Andreas Mershin et al., Self-assembled photosystem-I biophotovoltaics on nanostructured TiO2 and ZnO, Scientific Reports, 2012 [DOI: 10.1038/srep00234]