How to detect microvesicles in the bloodstream to diagnose and monitor brain cancer
November 13, 2012
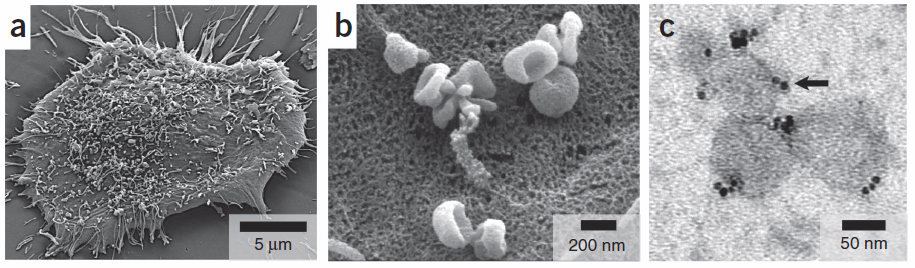
Human glioblastoma cancer cells (a) produce abundant microvesicles (b) in the bloodstream, which can be analyzed (c): the microvesicles (~80 nm) are attached to magnetic nanoparticles (MNPs) via an antibody. The MNPs, whic appear as black dots here (indicated by an arrow), are then detected by a miniature nuclear magnetic resonance system; the microvesicles are then separated and isolated (credit: Huilin Shao et al./Nature Medicine)
Diagnosing glioblastoma brain cancer is a real challenge for neurologists because they are deep in the brain and hard to test for. Now there’s a promising new solution.
A novel miniature diagnostic platform using nuclear magnetic resonance (NMR) technology that can detect minuscule cell particles known as microvesicles (shed by cancer cells) in a drop of blood has been developed by investigators at the Massachusetts General Hospital (MGH) Center for Systems Biology (CSB).
Background
Glioblastoma multiforme (GBM) is the most common and most aggressive brain cancer in humans. By the time it is diagnosed, patients typically have less than 15 months to live. One of the biggest challenges with this condition is accurate disease monitoring to establish whether patients are responding to treatment.
Currently, the only way to diagnose and monitor GBM is with biopsies and imaging tests, making long-term treatment monitoring difficult, invasive and impractical. To address this need, the CSB team sought to develop a simple blood test that could be used to easily monitor disease progression.
“About 30 or 40 years ago, people noticed something in the bloodstream that they initially thought was some kind of debris or ‘cell dust’,”explains Hakho Lee, PhD, of the CSB, and co-senior author of the study with Ralph Weissleder, MD, PhD, director of the CSB. “But it has recently become apparent that these vesicles shed by cells actually harbor the same biomarkers as their parent cells.”
Circulating tumor cells (CTCs) were thought of as a potential key to improved cancer diagnosis, but the problem with CTCs is that they are extremely rare, Lee says, “so finding them in the blood is like trying to find a needle in a haystack. Microvesicles on the other hand are abundant in the circulation and, unlike CTCs, are small enough to cross the blood/brain barrier, which means that they could be used to detect and monitor brain cancers.”
“The issue with microvesicles, however, is that they are very small, so there are not many technologies out there that can detect and molecularly profile them,” explains Lee. “That is where our new technology comes in.”
Detecting microvesicles
1. Antibody molecules are attached to the microvesicles and the magnetic nanoparticles (MNPs) are then attached to the antibody molecules.
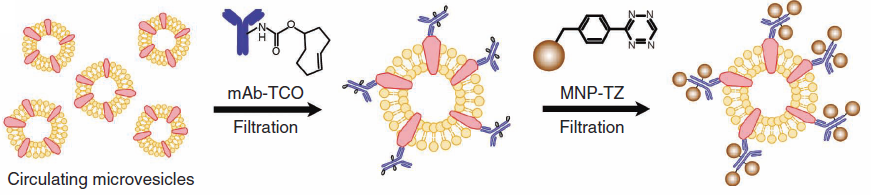
Two-step labeling procedure for MNP binding onto target proteins on microvesicles (credit: Huilin Shao et al./Nature Medicine)
2. A microfluidic microvesicle detection system routes the fluid past a coil, which detects the magnetic nanoparticles (similar to how MRI works), and the attached microvesicles are then isolated and captured.
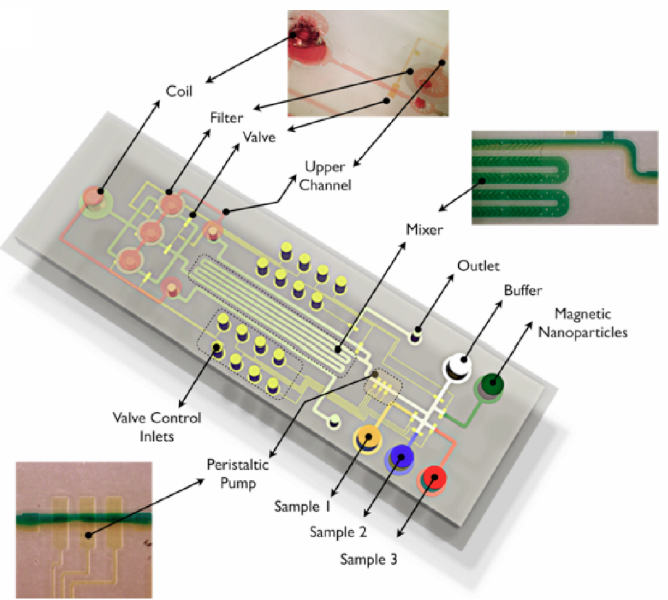
Microfluidic microvesicle detection system can load and test three samples individually
(credit: Huilin Shao et al./Nature Medicine)
The MGH researchers have now been able to reliably detect the tumor microvesicles in blood samples from mice that are bearing human GBM tumors — and eventually in samples from human GBM patients.
Compared with other gold-standard techniques, this new technology demonstrated excellent detection accuracy. Unlike other methods — which can be time-consuming and require much greater sample volumes as well as expertise to perform — NMR detection will be quick and simple, potentially providing almost instant results from a small blood sample right in a doctor’s office, the authors note.
“These microvesicles were found to be remarkably reliable biomarkers,” confirms Weissleder. “They are very stable and abundant and appear to be extremely sensitive to treatment effects. In both animals and human patients, we were able to monitor how the number of cancer-related microvesicles in the bloodstream changed with treatment,” explains Weissleder. “Even before an appreciable change in tumor size could be seen with imaging, we saw fewer microvesicles. It’s like they are a harbinger of treatment response.”
The MGH CSB team is currently extending this platform to other types of cancer and to other diseases such as bacterial infection. A number of clinical studies are currently ongoing, and others are in the planning stages, with the goal of eventually commercializing the technology.
Weissleder is a professor of Radiology and Lee an assistant professor at Harvard Medical School.