How to erase bad memories and enhance good ones
May 27, 2016
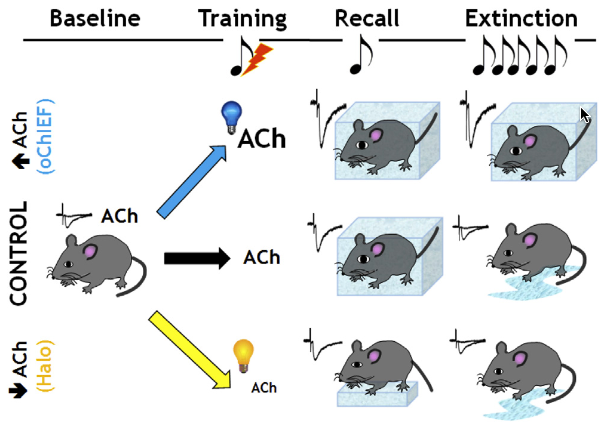
Mice normally freeze in position as a response to fear, as shown here under control condition (center row): fear conditioning induces freezing behavior in response (recall) to exposure to the conditioned stimulus (tone), but the freezing response normally decreases (extinction) following several days of multiple tone exposures (the mice get used to it). However, enhancing release of acetylcholine (blue light) to the amygdala during conditioned fear training resulted in continued freezing behavior 24 hours later and persisted over long periods of time (extinction). In contrast, reducing acetylcholine (yellow light) during the initial training period reduced the freezing behavior (during recall) and led to greater retention of the extinction learning (reduced freezing). (credit: Li Jiang et al./Neuron)
Imagine if people with dementia could enhance good memories or those with post-traumatic stress disorder could wipe out bad memories. A Stony Brook University research team has now taken a step toward that goal by manipulating one of the brain’s natural mechanisms for signaling involved in emotional memory: a neurotransmitter called acetylcholine.
The region of the brain most involved in emotional memory is thought to be the amygdala. Cholinergic neurons that reside in the base of the brain — the same neurons that appear to be affected early in cognitive decline — stimulate release of acetylcholine by neurons in the amygdala, which strengthens emotional memories.
Because fear is a strong and emotionally charged experience, Lorna Role, PhD, Professor and Chair of the Department of Neurobiology and Behavior, and colleagues used a fear-based memory model in mice to test the underlying mechanism of memory and the specific role of acetylcholine in the amygdala.
A step toward reversing post-traumatic stress disorder

Be afraid. Be very afraid. Optogenetic stimulation with blue light. (credit: Deisseroth Laboratory)
To achieve precise control, the team used optogenetics, a research method using light, to stimulate specific populations of cholinergic neurons in the amygdala during the experiments to release acetylcholine. As noted in previous studies reported on KurzweilAI, shining blue (or green) light on neurons treated with light-sensitive membrane proteins stimulates the neurons while shining yellow (or red) light inhibits (blocks) them.
So when the researchers used optogenetics with blue light to increase the amount of acetylcholine released in the amygdala during the formation of a traumatic memory, they found it greatly strengthened fear memory, making the memory last more than twice as long as normal.
But when they decreased acetylcholine signaling (using yellow light) in the amygdala from a traumatic experience — one that normally produces a fear response — they could actually extinguish (wipe out) the memory.
Role said the long-term goal of their research is to find ways — potentially independent of acetylcholine (or drug administration) — to enhance or diminish the strength of good memories and diminish the bad ones.
Their findings are published in the journal Neuron. The research was supported in part by the National Institutes of Health.
Abstract of Cholinergic Signaling Controls Conditioned Fear Behaviors and Enhances Plasticity of Cortical-Amygdala Circuits
We examined the contribution of endogenous cholinergic signaling to the acquisition and extinction of fear- related memory by optogenetic regulation of cholinergic input to the basal lateral amygdala (BLA). Stimulation of cholinergic terminal fields within the BLA in awake-behaving mice during training in a cued fear-conditioning paradigm slowed the extinction of learned fear as assayed by multi-day retention of extinction learning. Inhibition of cholinergic activity during training reduced the acquisition of learned fear behaviors. Circuit mechanisms underlying the behavioral effects of cholinergic signaling in the BLA were assessed by in vivo and ex vivo electrophysiological recording. Photostimulation of endogenous cholinergic input (1) enhances firing of putative BLA principal neurons through activation of acetylcholine receptors (AChRs), (2) enhances glutamatergic synaptic transmission in the BLA, and (3) induces LTP of cortical-amygdala circuits. These studies support an essential role of cholinergic modulation of BLA circuits in the inscription and retention of fear memories.