How to make instant carbon nanoparticles at home for cool biomedical uses
June 19, 2015
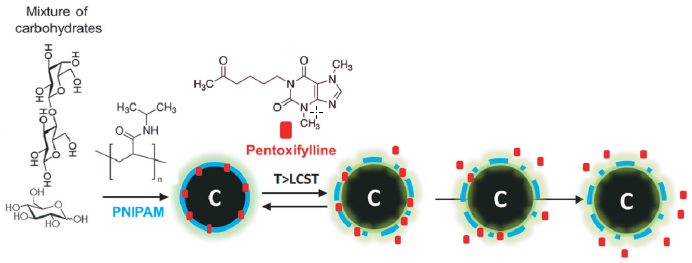
Luminescent carbon nanoparticles (a mixture of carbohydrates) were used for controlled drug delivery. Poly( N -isopropylacrylamide) (PNIPAM), a thermoresponsive (heat-sensitive) polymer, was chosen to encapsulate pentoxifylline drug (red) molecules. PNIPAM undergoes a phase transition at a higher temperature, releasing the drug molecule in a controlled fashion. (credit: P. Mukherjee et al./Small)
How would you like to produce carbon nanoparticles small enough to evade the body’s immune system, that reflect light in the near-infrared range for easy detection in the body, and even carry payloads of pharmaceutical drugs to targeted tissues — all in the privacy of your own home?
If so, well, University of Illinois bioengineering professors Dipanjan Pan and Rohit Bhargava have a DIY recipe for you.
Unlike other methods of making carbon nanoparticles — which require expensive equipment and purification processes that can take days — with the handy-dandy new approach, you can generates your own biomedical-class nanoparticles in a few hours, using store-bought molasses and honey. That and a pig.
The researchers report their big findings in the journal Small.
The DIY biomedical nanoparticles kitchen recipe
“If you have a microwave, honey, and molasses, you can pretty much make these particles at home,” Pan said. “You just mix these two together and cook it for a few minutes, and you get something that looks like char, but that is nanoparticles with high luminescence [they glow]. This is one of the simplest systems that we can think of. It is safe and highly scalable for eventual clinical use.”
These “next-generation” carbon nanospheres, or “luminescent carbon nanomaterials,” have several attractive properties, the researchers found:
- For imaging, they naturally scatter light in a manner that makes them easy to differentiate from human tissues, eliminating the need for added dyes or fluorescing molecules to help detect them in the body. The nanoparticles are coated with polymers that fine-tune their optical properties and their rate of degradation in the body.
- The polymers can also be loaded with drugs that are gradually released.
The nanoparticles also can be made less than eight nanometers in diameter (a human hair is 80,000 to 100,000 nanometers thick). “Our immune system fails to recognize anything under 10 nanometers,” Pan said. “So, these tiny particles are kind of camouflaged, I would say; they are hiding from the human immune system.”
Testing the nanoparticles: don’t try this at home
Unless you have a really unusual kitchen, this part will require a lab. Bhargava’s laboratory used vibrational spectroscopic techniques to identify the molecular structure of the nanoparticles and their cargo.
“Raman and infrared spectroscopy are the two tools that one uses to see molecular structure,” Bhargava said. “We think we coated this particle with a specific polymer and with specific drug-loading — but did we really? We use spectroscopy to confirm the formulation as well as visualize the delivery of the particles and drug molecules.”
The team found that the nanoparticles did not release the drug payload at room temperature, but at body temperature began to release the anti-cancer drug.
Here’s where the pig comes in. The team tested the therapeutic potential of the nanoparticles by loading them with an anti-melanoma drug and mixing them in a topical solution that was applied to pig skin.The researchers also determined which topical applications penetrated the skin to a desired depth.
In further experiments, the researchers found they could alter the infusion of the particles into melanoma cells by adjusting the polymer coatings. Imaging confirmed that the infused cells began to swell, a sign of impending cell death.
“This is a versatile platform to carry a multitude of drugs — for melanoma, for other kinds of cancers and for other diseases,” Bhargava said. “You can coat it with different polymers to give it a different optical response. You can load it with two drugs, or three, or four, so you can do multidrug therapy with the same particles.”
“By using defined surface chemistry, we can change the properties of these particles,” Pan said. “We can make them glow at a certain wavelength and also we can tune them to release the drugs in the presence of the cellular environment. That is, I think, the beauty of the work.”
Abstract of Tunable luminescent carbon nanospheres with well-defined nanoscale chemistry for synchronized imaging and therapy
In this work, we demonstrate the significance of defined surface chemistry in synthesizing luminescent carbon nanomaterials (LCN) with the capability to perform dual functions (i.e., diagnostic imaging and therapy). The surface chemistry of LCN has been tailored to achieve two different varieties: one that has a thermoresponsive polymer and aids in the controlled delivery of drugs, and the other that has fluorescence emission both in the visible and near-infrared (NIR) region and can be explored for advanced diagnostic modes. Although these particles are synthesized using simple, yet scalable hydrothermal methods, they exhibit remarkable stability, photoluminescence and biocompatibility. The photoluminescence properties of these materials are tunable through careful choice of surface-passivating agents and can be exploited for both visible and NIR imaging. Here the synthetic strategy demonstrates the possibility to incorporate a potent antimetastatic agent for inhibiting melanomas in vitro. Since both particles are Raman active, their dispersion on skin surface is reported with Raman imaging and utilizing photoluminescence, their depth penetration is analysed using fluorescence 3D imaging. Our results indicate a new generation of tunable carbon-based probes for diagnosis, therapy or both.