Imaging most of a worm’s brain activity at high resolution and in a single operation
September 11, 2013
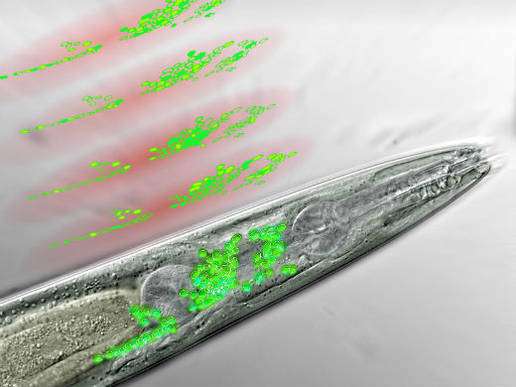
Frontal part of a nematode seen through a microscope. The neurons of the worm’s “brain” are in green. Above are discs of light generated by the microscope, scanning the brain area and recording the activity of certain neurons (artist’s interpretation) (Credit: IMP)
A new technique developed by Austrian scientists can record the activity of a worm’s brain with high temporal and spatial resolution, ultimately allowing for linking brain anatomy to brain function.
The worm in this study is nematode C. elegans, which has 302 neurons connected by roughly 8000 synapses. It is the only animal for which a complete nervous system has been anatomically mapped.
Researchers have so far focused on studying the activity of single neurons and small networks in the worm, but have not been able to establish a functional map of the entire nervous system.
This is mainly due to limitations in the imaging techniques they employ: the activity of single cells can be resolved with high precision, but simultaneously looking at the function of all neurons that comprise entire brains has been a major challenge.
Scientists at Vienna’s Research Institute of Molecular Pathology (IMP), the Max Perutz Laboratories (MFPL), and the Research Platform Quantum Phenomena & Nanoscale Biological Systems (QuNaBioS) of the University of Vienna have now developed a new technique based on “sculpting” the three-dimensional distribution of light in the brain.
C elegans nematode (credit: The Goldstein Lab)
With this new kind of microscopy, they are able to record the activity of 70% of the nerve cells in a worm’s head with high spatial and temporal resolution.
Visualizing the neurons also requires tagging them with a fluorescent protein that lights up when it binds to calcium, signaling the nerve cells’ activity.
“The neurons in a worm’s head are so densely packed that we could not distinguish them on our first images,” explains neurobiologist Tina Schrödel, co-first author of the study.
“Our solution was to insert the calcium sensor into the nuclei rather than the entire cells, thereby sharpening the image so we could identify single neurons.”
The new technique will open up the way for experiments that were not possible before. One of the questions that will be addressed is how the brain processes sensory information to “plan” specific movements and then executes them.