Master protein found to enhance both muscles and the brain
April 7, 2015
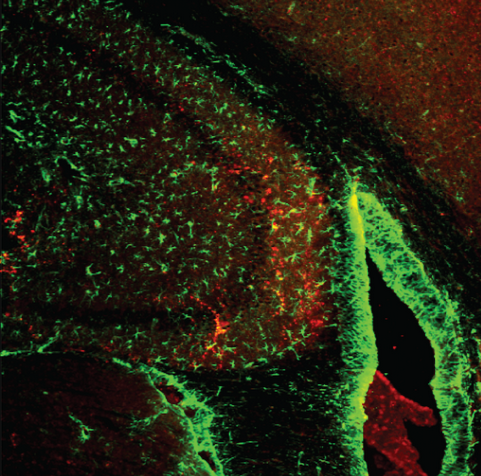
Salk researchers and collaborators discovered that physical and mental activities rely on a single metabolic protein, ERRγ, that controls the flow of blood and nutrients throughout the body. In this image, ERRγ is shown (stained red) in the hippocampus, the area of the brain largely responsible for memory. The new work could point to a way to enhance learning. (credit: Salk Institute)
Salk Institute for Biological Studies scientists and collaborators have discovered that physical and mental activities rely on a single metabolic protein called estrogen-related receptor gamma (ERRγ) that controls the flow of blood and nutrients throughout the body.
The new study could point to potential treatments in regenerative and developmental medicine as well as ways to address defects in learning and memory.
“This is all about getting energy where it’s needed to ‘the power plants’ in the body,” says Ronald Evans, director of Salk’s Gene Expression Laboratory and senior author of the new paper, published April 7 in the journal Cell Metabolism.
“The heart and muscles need a surge of energy to carry out exercise and neurons need a surge of energy to form new memories.” Energy for both muscles and brains, the scientists discovered, is controlled by the ERRγ protein.
Evans’ research group has previously studied the role of ERRγ in the heart and skeletal muscles. In 2011, they discovered that promoting ERRγ activity in the muscle of sedentary mice increased blood supply to their muscles and doubled their running capacity. ERRγ, they went on to show, turns on a whole host of muscle genes that convert fat to energy.
ERRy burns fat, but curiously, it’s also active in the hippocampus
Previously, ERRγ was known as a master metabolic switch that regulates a network of genes that in turn drives the body to burn fat. Although studies had also shown that ERRγ was active in the brain, researchers didn’t understand why — the brain burns sugar, not fat, and ERRγ was previously shown to only turn on fat-burning pathways. The researchers decided to look more closely at what the protein was doing in brain cells.
By first looking at isolated neurons, Evans’ group showed that ERRγ activates dozens of metabolic genes in the cells. Neurons that lacked ERRγ didn’t produce as much ATP — a cellular energy molecule — as those that had high levels of the protein. It turned out that the genes controlled by ERRγ in brain cells are those involved in burning sugar, not fat, for energy.
“We assumed that ERRγ did the same thing throughout the body,” says Evans. “But we learned that it’s adaptable to different metabolic roles in different organs.” ERRγ, they now believe, turns on fat-burning pathways in muscles and sugar-burning pathways in the brain.
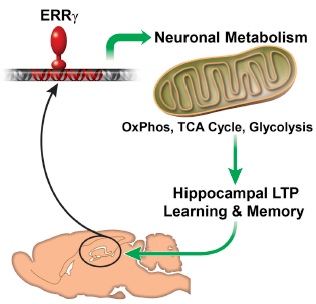
The estrogen-related receptor gamma (ERRg ) protein regulates a neural gene network promoting mitochondrial oxidative metabolism that is essential to meet the energy demand of spatial learning and memory formation. (credit: Liming Pei et al./Cell Metabolism)
Evans and his collaborators noticed that ERRγ in live mice was most active in the hippocampus — an area of the brain that can yield new brain cells, is involved in learning and memory and is known to require lots of energy. They wondered whether ERRγ had a direct role in learning and memory. By studying mice without ERRγ in their brain cells, they found a link.
Mice without the protein had normal vision, movement and balance, but were slower at learning how to swim through a water maze—and worse at remembering the maze on subsequent trials—compared to mice with normal levels of ERRγ.
The metabolic variable in learning and memory
“What we found is that mice that lack ERRγ are basically very slow learners,” says Liming Pei, lead and co-corresponding author of the paper. Varying levels of ERRγ could also be at the root of differences between how humans learn, he hypothesizes. “Everyone can learn, but some people learn and memorize more efficiently than others, and we now think there’s a metabolic difference to it.”
A better understanding of the metabolism of neurons could help point the way to improved treatments for learning and attention disorders. And possibly, revving up levels of ERRγ could even enhance learning, just as it enhances muscle function.
“What we’ve shown is that memories are really built on a metabolic scaffold,” says Evans. “And we think that if you want to understand learning and memory, you need to understand this metabolic scaffold.”
Researchers at the University of Pennsylvania, Northwestern University, the University of Sydney, and Ecole Polytechnique Federale de Lausanne were also involved in the work, which was supported by the Howard Hughes Medical Institute, the National Institutes of Health, the Leona M. and Harry B. Helmsley Charitable Trust, the Ellison Medical Foundation and Glenn Foundation for Medical Research, the Children’s Hospital of Philadelphia and the Penn Medicine Neuroscience Center.
Abstract of Dependence of Hippocampal Function on ERRγ-Regulated Mitochondrial Metabolism
Neurons utilize mitochondrial oxidative phosphorylation (OxPhos) to generate energy essential for survival, function, and behavioral output. Unlike most cells that burn both fat and sugar, neurons only burn sugar. Despite its importance, how neurons meet the increased energy demands of complex behaviors such as learning and memory is poorly understood. Here we show that the estrogen-related receptor gamma (ERRγ) orchestrates the expression of a distinct neural gene network promoting mitochondrial oxidative metabolism that reflects the extraordinary neuronal dependence on glucose. ERRγ−/− neurons exhibit decreased metabolic capacity. Impairment of long-term potentiation (LTP) in ERRγ−/− hippocampal slices can be fully rescued by the mitochondrial OxPhos substrate pyruvate, functionally linking the ERRγ knockout metabolic phenotype and memory formation. Consistent with this notion, mice lacking neuronal ERRγ in cerebral cortex and hippocampus exhibit defects in spatial learning and memory. These findings implicate neuronal ERRγ in the metabolic adaptations required for memory formation.