Miniature brain organoids made from patient skin cells reveal insights into autism
July 16, 2015
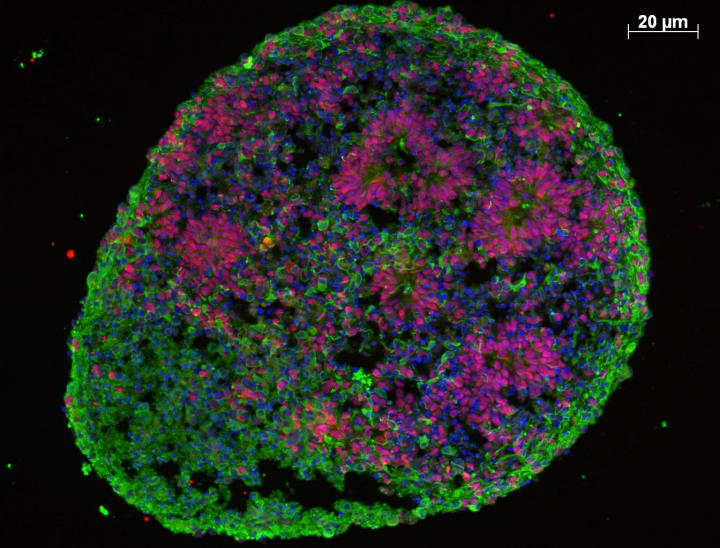
A human brain organoid showing complex internal organization, with immature proliferating cells (red) and a surrounding layer of maturing neurons (green) (credit: Jessica Mariani)
Taking a radical research approach to understanding autism, Yale School of Medicine researchers converted skin cells from autism patients into stem cells and then grew them into tiny brains in a dish — revealing unexpected mechanisms of the disease.
The study was published in an open-access paper today (July 16) in the journal Cell.
Most autism research has taken the approach of combing through patient genomes for mutations that may underlie the disorder and then using animal or cell-based models to study the genes and their possible roles in brain development. That has left more than 80% of autism cases with no clear genetic cause.
“Instead of starting from genetics, we’ve started with the biology of the disorder itself to try to get a window into the genome,” says senior author Flora Vaccarino the Harris Professor of Child Psychiatry and Professor or Neurobiology at Yale.
The clinical characteristics of autism are complex and wide-ranging, making the prospect of finding common underlying factors slim. So the researchers focused on the approximately one-fifth of autism patients that share a distinctive feature correlated with disease severity — an enlarged brain.
Unexpected neuron-type imbalance
The “brain organoids” that resulted are just a few millimeters about a fifth of a millimeter in diameter but mimic the basics of early human brain development, roughly corresponding to the first few months of gestation. When the researchers analyzed the patient organoids, they uncovered altered expression networks for genes controlling neuronal development.
The patient organoids showed an unexpected overproduction of inhibitory neurons that quiet down neural activity, while those that excite the partners they’re wired to were unaffected, leading to an imbalance in neuron type. Remarkably, by suppressing the expression of a single gene whose expression was abnormally increased in patient organoids, the authors were able to correct this bias, suggesting that it may be possible to intervene clinically to restore neuronal balance.
The authors are now using their data to home in on the difficult-to-find mutations or epigenetic changes responsible for the gene expression alterations and neuronal imbalance observed in the study. Efforts to extend their growth to later embryonic stages are also under way by a number of groups and will allow even further insights into the disease mechanisms.
“This study speaks to the importance of using human cells and using them in an assay that could bring a better understanding of the pathophysiology of autism and with that, possibly better treatments,” according to Vaccarino.
The success of the approach also suggests that similar methods might be used to gain important insights into other human developmental diseases that have until now been difficult to crack open.
Abstract of FOXG1-Dependent Dysregulation of GABA/Glutamate Neuron Differentiation in Autism Spectrum Disorders
Autism spectrum disorder(ASD) is a disorder of brain development. Most cases lack a clear etiology or genetic basis, and the difficulty of re-enacting human brain development has precluded understanding of ASD pathophysiology. Here we use three-dimensional neural cultures (organoids) derived from induced pluripotent stem cells (iPSCs) to investigate neurodevelopmental alterations in individuals with severe idiopathic ASD. While no known underlying genomic mutation could be identified, transcriptome and gene network analyses revealed upregulation of genes involved in cell proliferation, neuronal differentiation, and synaptic assembly. ASD-derived organoids exhibit an accelerated cell cycle and overproduction of GABAergic inhibitory neurons. Using RNA interference, we show that overexpression of the transcription factor FOXG1 is responsible for the overproduction of GABAergic neurons. Altered expression of gene network modules and FOXG1 are positively correlated with symptom severity. Our data suggest that a shift toward GABAergic neuron fate caused by FOXG1 is a developmental precursor of ASD.