Muscle-powered bio-bots walk on command
July 2, 2014
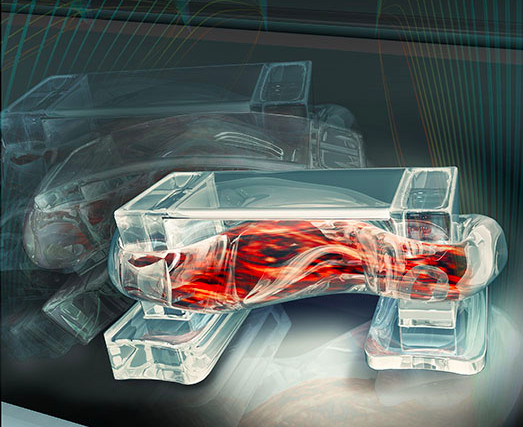
Tiny walking “bio-bots” are powered by muscle cells and controlled by an electric field (credit: University of Illinois)
Engineers at the University of Illinois at Urbana-Champaign have demonstrated a class of walking “bio-bots” powered by muscle cells and controlled with electrical pulses, giving researchers unprecedented command over their function.
The group published its work in the Proceedings of the National Academy of Science (open access).
“Biological actuation driven by cells is a fundamental need for any kind of biological machine you want to build,” said study leader Rashid Bashir, Abel Bliss Professor and head of bioengineering at the U. of I.
Bashir’s group has been a pioneer in designing and building bio-bots, less than a centimeter in size, made of flexible 3-D printed hydrogels and living cells. Previously, the group demonstrated bio-bots that “walk” on their own, powered by beating heart cells from rats.
However, heart cells constantly contract, denying researchers control over the bot’s motion. This makes it difficult to use heart cells to engineer a bio-bot that can be turned on and off, sped up or slowed down.
The new bio-bots are powered by a strip of skeletal muscle cells that can be triggered by an electric pulse. This gives the researchers a simple way to control the bio-bots and opens the possibilities for customize bio-bots for specific applications.
“Skeletal muscles cells are very attractive because you can pace them using external signals,” Bashir said. “For example, you would use skeletal muscle when designing a device that you wanted to start functioning when it senses a chemical or when it received a certain signal. To us, it’s part of a design toolbox. We want to have different options that could be used by engineers to design these things.”
The design is inspired by the muscle-tendon-bone complex found in nature. There is a backbone of 3-D printed hydrogel, strong enough to give the bio-bot structure but flexible enough to bend like a joint. Two posts serve to anchor a strip of muscle to the backbone, like tendons attach muscle to bone, but the posts also act as feet for the bio-bot.
A bot’s speed can be controlled by adjusting the frequency of the electric pulses. A higher frequency causes the muscle to contract faster, thus speeding up the bio-bot’s progress, as seen in this video:
From the lab of Rashid Bashir. Image: Janet Sinn-Hamlon, DesignGroup@VetMed. Animation: Doug Litteken. Video: Caroline Cvetkovic and Ritu Raman.
“This work represents an important first step in the development and control of biological machines that can be stimulated, trained, or programmed to do work,” said said graduate student Caroline Cvetkovic, co-first author of the paper. “It’s exciting to think that this system could eventually evolve into a generation of biological machines that could aid in drug delivery, surgical robotics, ‘smart’ implants, or mobile environmental analyzers, among countless other applications.”
Next, the researchers will work to gain even greater control over the bio-bots’ motion, like integrating neurons so the bio-bots can be steered in different directions with light or chemical gradients. On the engineering side, they hope to design a hydrogel backbone that allows the bio-bot to move in different directions based on different signals.
Thanks to 3-D printing, engineers can explore different shapes and designs quickly. Bashir and colleagues even plan to integrate a unit into undergraduate lab curriculum so that students can design different kinds of bio-bots.
The National Science Foundation supported this work through a Science and Technology Center (Emergent Behavior of Integrated Cellular Systems) grant, in collaboration with the Massachusetts Institute of Technology, the Georgia Institute of Technology and other partner institutions.
Abstract of Proceedings of the National Academy of Sciences paper
Combining biological components, such as cells and tissues, with soft robotics can enable the fabrication of biological machines with the ability to sense, process signals, and produce force. An intuitive demonstration of a biological machine is one that can produce motion in response to controllable external signaling. Whereas cardiac cell-driven biological actuators have been demonstrated, the requirements of these machines to respond to stimuli and exhibit controlled movement merit the use of skeletal muscle, the primary generator of actuation in animals, as a contractile power source. Here, we report the development of 3D printed hydrogel “bio-bots” with an asymmetric physical design and powered by the actuation of an engineered mammalian skeletal muscle strip to result in net locomotion of the bio-bot. Geometric design and material properties of the hydrogel bio-bots were optimized using stereolithographic 3D printing, and the effect of collagen I and fibrin extracellular matrix proteins and insulin-like growth factor 1 on the force production of engineered skeletal muscle was characterized. Electrical stimulation triggered contraction of cells in the muscle strip and net locomotion of the bio-bot with a maximum velocity of ∼156 μm s−1, which is over 1.5 body lengths per min. Modeling and simulation were used to understand both the effect of different design parameters on the bio-bot and the mechanism of motion. This demonstration advances the goal of realizing forward-engineered integrated cellular machines and systems, which can have a myriad array of applications in drug screening, programmable tissue engineering, drug delivery, and biomimetic machine design.