Nano-movies of biomolecules
December 10, 2014
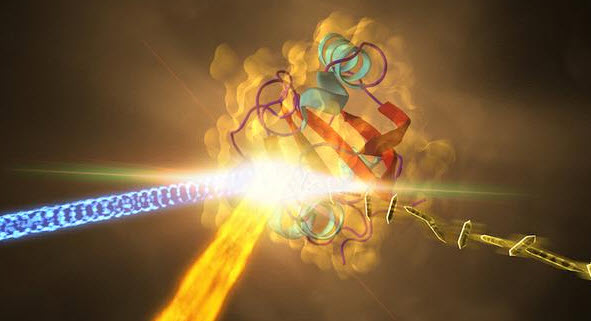
Samples of a crystallized protein (right) called photoactive yellow protein (PYP) are exposed to blue light (coming from left) to trigger shape changes, then were jetted into the path of SLAC’s LCLS X-ray laser beam (fiery beam from bottom left). Diffraction patterns created when the X-ray laser hit the crystals allowed scientists to recreate the 3D structure of the protein (center) and determine how light exposure changes its shape. (Credit: SLAC National Accelerator Laboratory)
An international team led by Prof. Marius Schmidt from the University of Wisconsin-Milwaukee has imaged a light-sensitive biomolecule with an X-ray laser at unprecedented atomic spatial resolution and ultrafast temporal (time) resolution, as the scientists write in the journal Science.
The researchers used the photoactive yellow protein (PYP) as a model system. PYP is a receptor for blue light that is part of the photosynthetic machinery in certain bacteria. When it catches a blue photon, it cycles through various intermediate structures as it harvests the energy of the photon, before it returns to its initial state. Most steps of this PYP photocycle have been well studied, making it an excellent candidate for validating a new method.
For their ultra-fast snapshots of the PYP dynamics, the scientists first produced tiny crystals of PYP molecules, most measuring less than 0.01 millimeters across. The microcrystals’ photocycle was kicked off by blue laser pulse and then the microcrystals were sprayed into the focus of the world’s most powerful X-ray laser, the LCLS at the SLAC National Accelerator Laboratory.
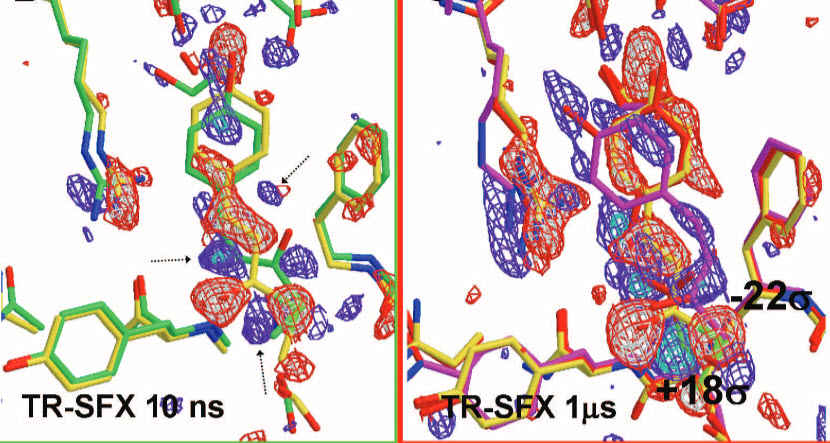
Snapshots of X-ray diffraction patterns at two different time steps (at 10 nanoseconds and at 1 microsecond) in the PYP protein’s photocycle (credit: Jason Tenboer et al./Science)
The extremely short, intense X-ray flashes of the LCLS allowed the researchers to watch how PYP changes its shape at different time steps in the photocycle, by taking snapshot X-ray diffraction patterns.
With a resolution of 0.16 nanometers, these are the most detailed images of a biomolecule ever made with an X-ray laser. The diameter of the smallest atom, hydrogen, is about 0.1 nanometers.
Also, thanks to the high temporal resolution, the X-ray laser could, in principle, study steps in the cycle that are shorter than 1 picosecond (a trillionth of a second) — too fast to be caught with previous techniques. The ultrafast snapshots can be assembled into a movie, showing the dynamics in ultra slow-motion.
“This is a real breakthrough,” said co-author Prof. Henry Chapman from the Center for Free-Electron Laser Science at DESY in Germany. “Our study is opening the door for time-resolved studies of dynamic processes with atomic resolution.”
Compared to other methods, X-ray lasers offer two major advantages for the investigation of ultrafast dynamics of molecules: the most brilliant X-ray flashes on earth and femtosecond time resolution. A femtosecond is a quadrillionth of a second (or one thousandth of a picosecond). While 40 femtosecond X-ray flashes were used for this experiment, the pulse duration can be made even shorter — down to just a few femtoseconds.
“You need a short pulse to resolve the steps of these fast processes,” says co-author Dr. Anton Barty, also from DESY. “The short flashes also overcome the problem of damaging the often delicate samples with the intense X-rays.” Although the powerful pulses usually vaporize the sample, they are so short that they produce a high-quality diffraction signal on the detector before the sample disintegrates. This principle, called diffraction before destruction, was proven a few years ago by an international collaboration led by DESY.
The team included researchers from the University of Wisconsin-Milwaukee, Arizona State University, SLAC National Accelerator Center, Lawrence Livermore National Laboratory, DESY, University of New York Buffalo, University of Chicago and Imperial College London.
Abstract of Time-resolved serial crystallography captures high-resolution intermediates of photoactive yellow protein
Serial femtosecond crystallography using ultrashort pulses from x-ray free electron lasers (XFELs) enables studies of the light-triggered dynamics of biomolecules. We used microcrystals of photoactive yellow protein (a bacterial blue light photoreceptor) as a model system and obtained high-resolution, time-resolved difference electron density maps of excellent quality with strong features; these allowed the determination of structures of reaction intermediates to a resolution of 1.6 angstroms. Our results open the way to the study of reversible and nonreversible biological reactions on time scales as short as femtoseconds under conditions that maximize the extent of reaction initiation throughout the crystal.