Nanoparticles cross blood-brain barrier, enhance medication delivery and MRI performance
May 2, 2012
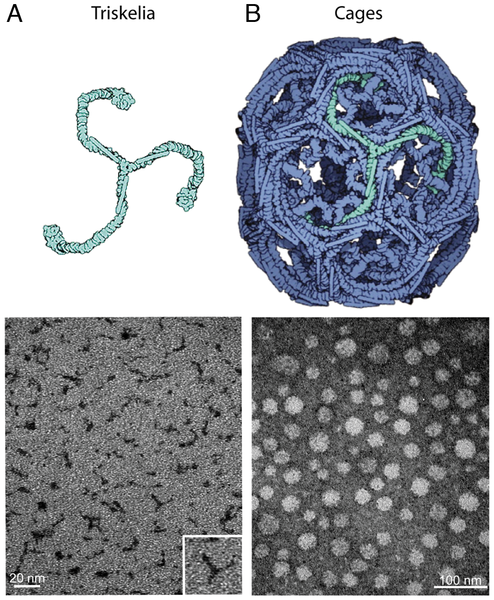
(A) A clathrin triskelion (light green) with attached Gd-DTPA-ITC negatively stained with 1% uranyl acetate. (B) Clathrin cage lattice (blue) self-assembled from clathrin triskelia. The TEM images showsclathrin cages with attached Gd-DTPA-ITC negatively stained with 1% uranyl acetate and clathrin cage-formed hexagonal barrels. (Credit: Gordana D. Vitaliano et al./PLoS ONE)
Researchers at Harvard-affiliated McLean Hospital have developed a new category of non-toxic, protein-based “green” nanoparticles that can non-invasively cross the blood brain barrier and transport various types of drugs.
In an article published May 1, 2012 online in PLoS ONE, Gordana Vitaliano, MD, director of the Brain Imaging NaNoTechnology Group at the McLean Hospital Imaging Center, reported that clathrin protein, a ubiquitous protein found in human, animal, plant, bacteria and fungi cells, can be modified for use as a nanoparticle for in-vivo studies.
“Clathrin has never been modified for use in vivo and offers many new and interesting possibilities for delivering drugs and medical imaging agents into the brain”, said Vitaliano.
Clathrin is the body’s primary delivery vehicle responsible for delivering many different types of molecules into cells. Vitaliano suggested that the protein’s naturally potent transport capabilities might be put to practical medical use for drug delivery and medical imaging.
“This study provides a new insight into utilizing bioengineered clathrin protein as a novel nanoplatform that passes the blood brain barrier,” said Vitaliano, who successfully attached different fluorescent labels, commonly used in imaging, to functionalize clathrin nanoparticles.
“We were able to show that the clathrin nanoparticles could be non-invasively delivered to the central nervous system (CNS) in animals.”
Of major importance for future clinical applications, Vitaliano also showed that clathrin crossed and/or bypassed the blood-brain barrier without enhancers or modifications, unlike other nanoparticles. These findings open the door to exploring new and important CNS medical applications.
Improved MRI performance
One important medical application for clathrin nanoparticles would be Magnetic Resonance Imaging (MRI). Gadolinium contrast agents are often used to improve MRI performance. In one configuration, Vitaliano found that functionalized clathrin nanoparticles performed 8,000 times better than an FDA-approved MRI contrast agent (gadopentetate dimeglumine).
“Stated another way, it means 8,000 times less gadolinium might be required for achieving good MRI results. Because very low gadolinium concentrations would be required for MRI, it could significantly decrease gadolinium toxicity, which is an important issue,” explained Vitaliano. “Clathrin transported gadolinium is therefore among the most potent, biocompatible contrast agents available.”
These results in two different applications showed that clathrin offers substantial functionalization and transport flexibility. Purified clathrin nanoparticles could serve as an appealing alternative to other medical nanoplatforms, such as dendrimers, nanogels, solid lipid nanospheres, liposomes, and the like.
Given the critical need for new types of CNS drug transport capabilities, Vitaliano said her work would likely be of interest to researchers involved in neuroimaging and neuroscience, but also to radiologists, bioengineers, chemists, physicists, material scientists, biomedical researchers, and other researchers active at the frontiers of imaging and drug delivery.
Looking ahead, Vitaliano noted that her findings may also facilitate other studies for examining signaling pathways in different diseases that rely in whole or in part on clathrin transport, and thus may have a substantial impact in multiple fields.
Ref.: Gordana D. Vitaliano et al., New Clathrin-Based Nanoplatforms for Magnetic Resonance Imaging, PLoS ONE, 2012, DOI: 10.1371/journal.pone.0035821 (open access)