Nanoshell shields foreign enzymes used to starve cancer cells from immune system
July 3, 2014

The shell’s pores are too small for the enzyme to escape but big enough for diffusion of amino acids that feed cancer cells in and out of the particle. The enzymes remain trapped inside where they deplete any amino acids that enter. (Credit: Inanc Ortac)
Nanoengineers at the University of California, San Diego have developed a nanoshell to protect foreign enzymes used to starve cancer cells as part of chemotherapy.
Enzymes are naturally smart machines that are responsible for many complex functions and chemical reactions in biology. However, despite their huge potential, their use in medicine has been limited by the immune system, which is designed to attack foreign intruders.
For example, doctors have long relied on an enzyme called asparaginase to starve cancer cells as a patient undergoes chemotherapy. But because asparaginase is derived from a nonhuman organism, E. Coli, it is quickly neutralized by the patient’s immune system and sometimes produces an allergic reaction.
In animal studies with asparaginase, and other therapeutic enzymes, the research team found that their porous hollow nanoshell effectively shielded enzymes from the immune system, giving them time to work.
Asparaginase works by reacting with amino acids that are an essential nutrient for cancer cells. The reaction depletes the amino acid, depriving the abnormal cells from the nutrients they need to proliferate.
“Ours is a pure engineering solution to a medical problem,” said Inanc Ortac (Ph.D. ’13), who developed the technology as part of his doctoral research in the laboratory of nanoengineering professor Sadik Esener at UC San Diego Jacobs School of Engineering.
A filter in the bloodstream
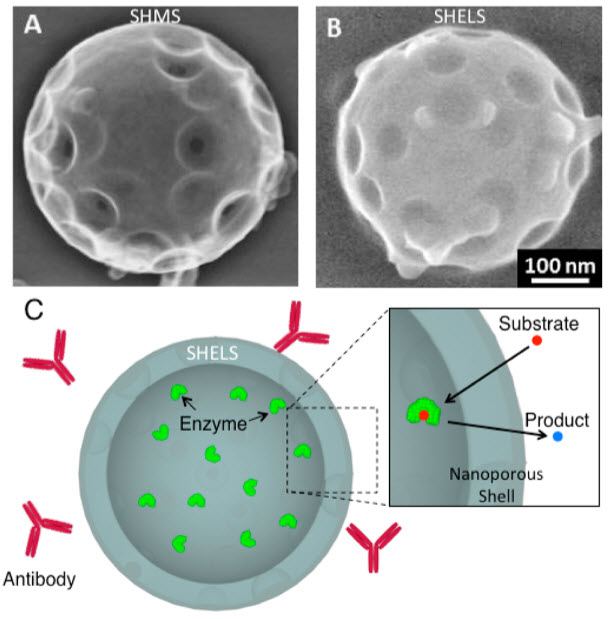
Scanning electron micrographs of (A) Synthetic hollow mesoporous nanospheres (SHMS) and (B) Synthetic hollow enzyme loaded nanospheres (SHELS). Scale bar refers to both (A) and (B). (C) Enzymes encapsulated within the hollow core of SHELS cannot escape. As depicted in the inset, showing the blowup of a section of SHELS, the small molecule substrate (red dots) can diffuse through the nanoporous shell, interact with the enzyme, and diffuse out of modified SHELS (blue dots). (Credit: Inanc Ortac et al./Nano Letters)
The nanoshell acts like a filter in the bloodstream. The enzymes are loaded into the nanoparticle very efficiently through pores on its surface and later encapsulated with a shell of nanoporous silica. The shell’s pores are too small for the enzyme to escape but big enough for diffusion of amino acids that feed cancer cells in and out of the particle. The enzymes remain trapped inside where they deplete any amino acids that enter.
“This is a platform technology that may find applications in many different fields. Our starting point was solving a problem for cancer therapeutics,” said Ortac.
Ortac is currently serving as the chief technology officer of DevaCell, a local start-up which licensed the technology and is working to commercialize it under the name Synthetic Hollow Enzyme Loaded nanoShells or SHELS.
The work is featured on the June 2014 cover of the journal Nano Letters. The research was supported by the National Cancer Institute.
UCSD Jacobs School of Engineering Department of Bioengineering and UC San Diego Moores Cancer Center | Silica Nanoparticles for Enzyme Delivery in Cancer Treatment, Ya-San Yeh, Sadik C. Esener
Abstract of Nano Letters paper
Although enzymes of nonhuman origin have been studied for a variety of therapeutic and diagnostic applications, their use has been limited by the immune responses generated against them. The described dual-porosity hollow nanoparticle platform obviates immune attack on nonhuman enzymes paving the way to in vivo applications including enzyme-prodrug therapies and enzymatic depletion of tumor nutrients. This platform is manufactured with a versatile, scalable, and robust fabrication method. It efficiently encapsulates macromolecular cargos filled through mesopores into a hollow interior, shielding them from antibodies and proteases once the mesopores are sealed with nanoporous material. The nanoporous shell allows small molecule diffusion allowing interaction with the large macromolecular payload in the hollow center. The approach has been validated in vivo using l-asparaginase to achieve l-asparagine depletion in the presence of neutralizing antibodies.