Neurons illuminate as they fire, may open new ways to trace brain signals
December 5, 2011
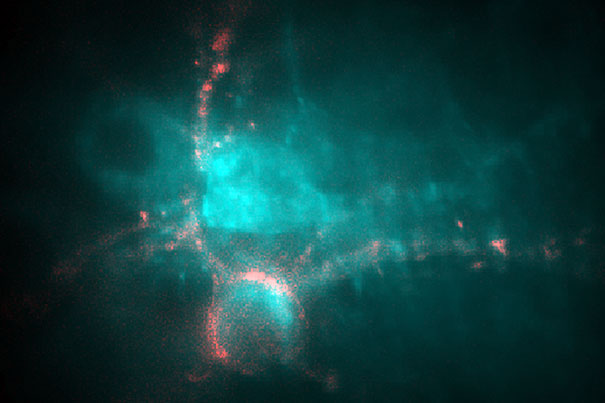
In a culture of neurons genetically modified to express a protein derived from a Dead Sea microorganism, the fluorescence of the cells depends on the voltage across the cell membrane. An increase in voltage in the cell in pink caused its fluorescence to stand out against the background of other cells. (Credit: Adam Cohen)
In a scientific first that could shed light on how signals travel in the brain and on the effects of learning on neural pathways, Harvard scientists have created genetically altered neurons that light up across a cell as they fire, eliminating the need to insert electrodes in each neuron.
The work may also lead to speedier drug development.
Led by Adam Cohen, John L. Loeb Associate Professor of the Natural Sciences, the researchers used a gene from a Dead Sea microorganism. The gene produced a protein that fluoresces when exposed to the electrical signal in a neuron, allowing scientists to trace the propagation of signals through the cell.
How to light up neurons
Cohen and his team infected brain cells that had been cultured in the lab with a genetically altered virus that contained the protein-producing gene. Once infected, the cells began manufacturing the protein, allowing them to light up.
When a neuron fires, its voltage reverses for a very short time, about a thousandth of a second, he explained. “This brief spike in voltage travels down the neuron and then activates other neurons downstream. Our protein is sitting in the [outside] membrane of the neurons, so as that pulse washes over the proteins, they light up, giving us an image of the neurons as they fire.”
We can now see how these signals spread through the neuronal network, said Cohen. “We can study the speed at which the signal spreads, and if it changes as the cells undergo changes. We may someday even be able to study how these signals move in living animals.”
New understanding of how electrical signals move in the brain
The research has the potential to revolutionize scientists’ understanding of how electrical signals move through the brain, as well as other tissues, Cohen said.
“Before, the best way to make a measurement of the electrical activity in a cell was to stick a little electrode into it and record the results on a volt meter,” he said. “The issue, however, was that you were only measuring the voltage at one point, you weren’t seeing a spatial map of how signals propagate. Now, we will be able to study how the signal spreads, whether it moves through all neurons at the same speed, and even how signals change if the cells are undergoing something akin to learning.”
Another limitation of using electrodes, Cohen said, is that the technique tends to kill the cells relatively quickly, making it impossible to study processes that take place over time. His new approach opens the door to studying the effects of growth and development on neurons; it could also lead to insights on stem cell development.
Being able to track the electrical pathways in cells holds practical applications, Cohen said, particularly when it comes to the development of new drugs or other therapies.
“Many, many drugs target ion channels, which are important proteins in governing the activity of the heart and brain,” he said. “Right now, if you want to test a compound designed to activate or inactivate a particular ion channel, you have to culture the cell, test it with an electrode, then add the drug and see what happens. This is an extremely slow process — it typically takes an hour or two for each data point.
“Now that we can do it optically in the microscope, we can test the efficacy of a drug on a cell in a few seconds. Instead of testing one compound or 10 compounds, we can try to test thousands or even hundreds of thousands. We can test different conditions, different mixtures — it will increase the throughput for testing new drugs.”
The process may even open new research avenues in the study of genetic conditions ranging from depression to heart disease.
Researchers can culture cells in the lab that are genetically identical to those of a patient known to carry a genetic predisposition to a particular condition, then study how signals move through those cells.
Ref.: Joel M Kralj et al., Optical recording of action potentials in mammalian neurons using a microbial rhodopsin, Nature Methods, 2011 [doi:10.1038/nmeth.1782]