Neuroscientists reverse Alzheimer’s disease in mice
February 19, 2018
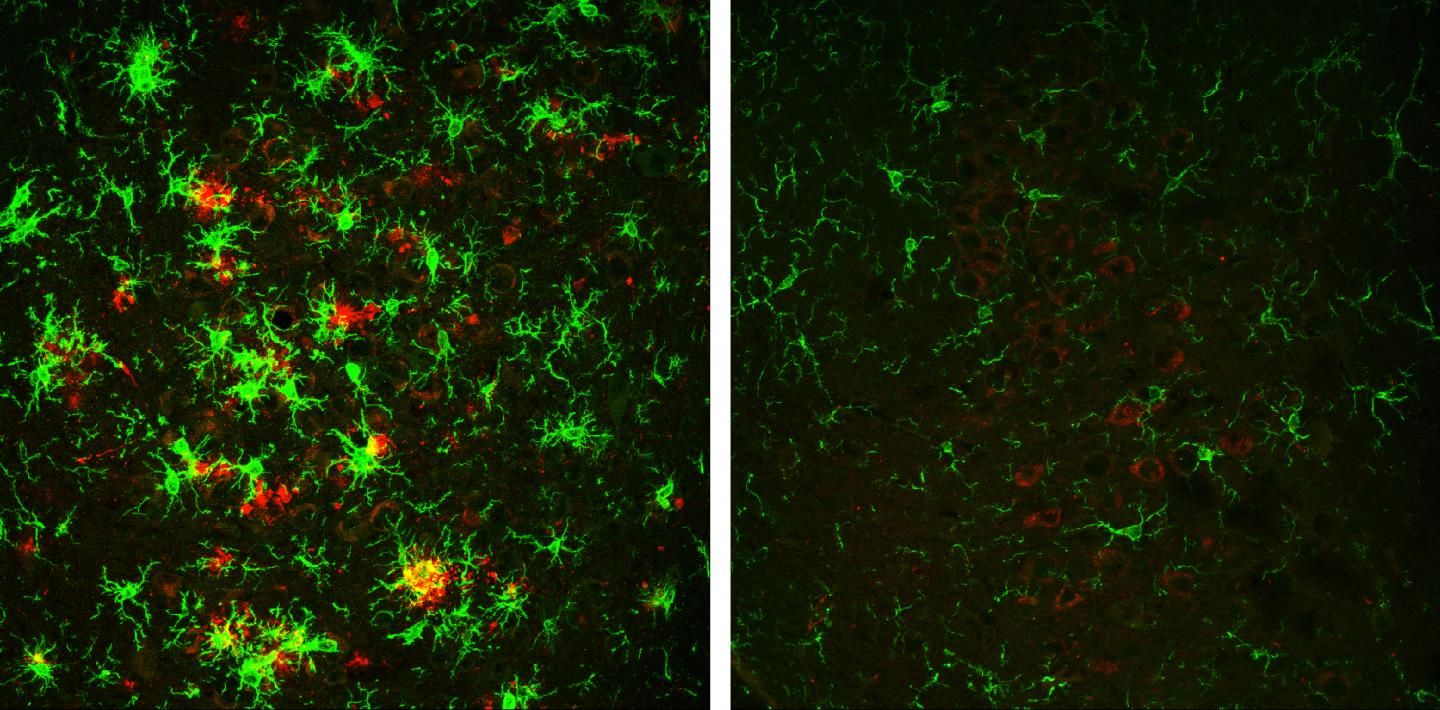
The brain of a 10-month-old mouse with Alzheimer’s disease (left) is full of amyloid plaques (red). These hallmarks of Alzheimer’s disease are reversed in animals that have gradually lost the BACE1 enzyme (right). (credit: Hu et al., 2018)
Researchers from the Cleveland Clinic Lerner Research Institute have completely reversed the formation of amyloid plaques in the brains of mice with Alzheimer’s disease by gradually depleting an enzyme called BACE1. The procedure also improved the animals’ cognitive function.
The study, published February 14 in the Journal of Experimental Medicine, raises hopes that drugs targeting this enzyme will be able to successfully treat Alzheimer’s disease in humans.
Background: Serious side effects
One of the earliest events in Alzheimer’s disease is an abnormal buildup of beta-amyloid peptide, which can form large, amyloid plaques in the brain and disrupt the function of neuronal synapses. The BACE1 (aka beta-secretase) enzyme helps produce beta-amyloid peptide by cleaving (splitting) amyloid precursor protein (APP). So drugs that inhibit BACE1 are being developed as potential Alzheimer’s disease treatments. But that’s a problem because BACE1 also controls many important neural processes; accidental cleaving of other proteins instead of APP could lead these drugs to have serious side effects. For example, mice completely lacking BACE1 suffer severe neurodevelopmental defects.
A genetic-engineering solution
To deal with the serious side effects, the researchers generated mice that gradually lose the BACE1 enzyme as they grow older. These mice developed normally and appeared to remain perfectly healthy over time. The researchers then bred these rodents with mice that start to develop amyloid plaques and Alzheimer’s disease when they are 75 days old.
The resulting offspring’s BACE1 levels were approximately 50% lower than normal (but also formed plaques at this age). However, as these mice continued to age and lose BACE1 activity, there were lower beta-amyloid peptide levels and the plaques began to disappear. At 10 months old, the mice had no plaques in their brains. Loss of BACE1 also improved the learning and memory of mice with Alzheimer’s disease.
“To our knowledge, this is the first observation of such a dramatic reversal of amyloid deposition in any study of Alzheimer’s disease mouse models,” says senior author Riqiang Yan, who will become chair of the department of neuroscience at the University of Connecticut this spring.
Decreasing BACE1 activity also reversed other hallmarks of Alzheimer’s disease, such as activation of microglial cells and the formation of abnormal neuronal processes.
However, the researchers also found that depletion of BACE1 only partially restored synaptic function, suggesting that BACE1 may be required for optimal synaptic activity and cognition.
“Our study provides genetic evidence that preformed amyloid deposition can be completely reversed after sequential and increased deletion of BACE1 in the adult,” says Yan. “Our data show that BACE1 inhibitors have the potential to treat Alzheimer’s disease patients without unwanted toxicity. Future studies should develop strategies to minimize the synaptic impairments arising from significant inhibition of BACE1 to achieve maximal and optimal benefits for Alzheimer’s patients.”
Abstract of BACE1 deletion in the adult mouse reverses preformed amyloid deposition and improves cognitive functions
BACE1 initiates the generation of the β-amyloid peptide, which likely causes Alzheimer’s disease (AD) when accumulated abnormally. BACE1 inhibitory drugs are currently being developed to treat AD patients. To mimic BACE1 inhibition in adults, we generated BACE1 conditional knockout (BACE1fl/fl) mice and bred BACE1fl/fl mice with ubiquitin-CreER mice to induce deletion of BACE1 after passing early developmental stages. Strikingly, sequential and increased deletion of BACE1 in an adult AD mouse model (5xFAD) was capable of completely reversing amyloid deposition. This reversal in amyloid deposition also resulted in significant improvement in gliosis and neuritic dystrophy. Moreover, synaptic functions, as determined by long-term potentiation and contextual fear conditioning experiments, were significantly improved, correlating with the reversal of amyloid plaques. Our results demonstrate that sustained and increasing BACE1 inhibition in adults can reverse amyloid deposition in an AD mouse model, and this observation will help to provide guidance for the proper use of BACE1 inhibitors in human patients.