New cryopreservation procedure wins Brain Preservation Prize
February 9, 2016
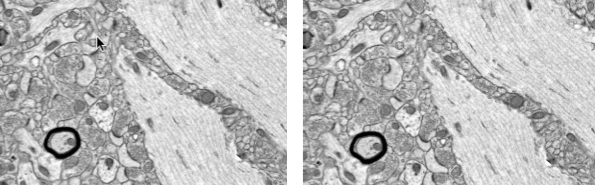
(Left): Control rabbit brain, showing neuropil near the CA1 band in the hippocampus. (Right): Vitrified rabbit brain, same location. Synapses, vesicles, and microfilaments are clear. The myelinated axon shows excellent preservation. (credit: Robert L. McIntyre and Gregory M. Fahy/Cryobiology)
The Brain Preservation Foundation (BPF) has announced that a team at 21st Century Medicine led by Robert McIntyre, PhD., has won the Small Mammal Brain Preservation Prize, which carries an award of $26,735.
The Small Mammalian Brain Preservation Prize was awarded after the determination that the protocol developed by McIntyre, termed Aldehyde-Stabilized Cryopreservation, was able to preserve an entire rabbit brain with well-preserved ultrastructure, including cell membranes, synapses, and intracellular structures such as synaptic vesicles (full protocol here).
The judges for the prize were Kenneth Hayworth, PhD., Brain Preservation Foundation President and neuroscientist at the Howard Hughes Medical Institute; and Prof. Sebastian Seung, PhD., Princeton Neuroscience Institute and Computer Science Department.
First preservation of the connectome
“This is a milestone in the development of brain preservation techniques: it is the first time that the preservation of the connectome has been demonstrated in a whole brain (prior to this only small parts of brains have been preserved to this level of detail),” said the BPF announcement.
“Current models of the brain suggest that the connectome contains all the information necessary for personal identity (i.e., memory and personality). This technique is not the same as conventional cryonics (rapidly freezing the brain), which has never demonstrated preservation of the ultrastructure of the brain. Thus the winning of this prize represents a significant advance in the methods available for large scale studies of the connectome and could lead to procedures that preserve a complete human brain.

Kenneth Hayworth (KH) (President of the Brain Preservation Foundation (BPF)) and Michael Shermer (member of BPF advisory board) witnessed (on Sept. 25, 2015) the full Aldehyde Stabilized Cryopreservation surgical procedure performed on this rabbit at the laboratories of 21 Century Medicine under the direction of 21CM lead researcher Robert McIntyre. This included the live rabbit’s carotid arteries being perfused with glutaraldehyde and subsequent perfusion with cryoprotectant agent (CPA). KH witnessed this rabbit brain being put in -135 degrees C storage, removal from storage the following day (verifying that it had vitrified solid), and KH witnessed all subsequent tissue processing steps involved in the evaluation process. (credit: The Brain Preservation Foundation)
“The key breakthrough was the rapid perfusion of a deadly chemical fixative (glutaraldehyde) through the brain’s vascular system, instantly stopping metabolic decay and fixing all proteins in place by covalent crosslinks. This stabilized the tissue and vasculature so that cryoprotectant could be perfused at an optimal temperature and rate. The result was an intact rabbit brain filled with such a high concentration of cryoprotectants that it could be stored as a solid ‘vitrified’ block at a temperature of -135 degrees Celsius.”
Winning the award also required that the procedure be published in a peer reviewed scientific publication. McIntyre satisfied this requirement and published the protocol in an open-access paper in the Journal of Cryobiology.
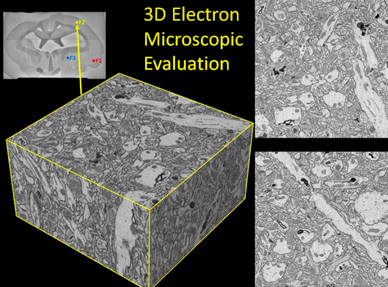
3D microscope evaluation of the rabbit brain tissue preservation (credit: Brain Preservation Foundation)
The Brain Preservation Foundation plans to continue to promote the idea that brain preservation following legal death, by using scientifically validated techniques, is a reasonable choice for consenting individuals to make. Focus now shifts to the final Large Mammal phase of the contest, which requires an intact pig brain to be preserved with similar fidelity in a manner that could be directly adapted to terminal patients in a hospital setting.
The 21st Century Medicine team has recently submitted to the BPF such a preserved pig brain for official evaluation. Lead researcher Robert McIntyre has started Nectome to further develop this method.
“Of course, [the demonstrated brain preservation procedure] is only useful if you think all the relevant information is preserved in the fixation,” said Anders Sandberg, PhD., of the Future of Humanity Institute/Oxford Martin School. “Protein states and small molecule chemical information may be messed up.”
GPA | Will You Preserve Your Brain?
Background and significance (statement by BPF)
Proponents of cryonics have long sought a technique that could put terminal patients into longterm stasis, the goal being a form of medical time travel in which patients are stabilized against decay with the hope of being biologically revived and cured by future technologies. Despite decades of research, this goal of reversible cryopreservation remains far out of reach — too much damage occurs during the cryopreservation itself.
This has led a new generation of researchers to focus on a more achievable and demonstrable goal–preservation of brain structure only. Specifically preservation of the delicate pattern of synaptic connections (the “connectome”) which neuroscience contends encodes a person’s memory and identity. Instead of biological revival, these new researchers often envision a future “synthetic revival” comprising nanometer-scale scanning of the preserved brain to serve as the basis for mind uploading.
This shift in focus toward “synthetic” revival has completely transformed the cryonics debate, opening up new avenues of research and bringing it squarely within the purview of today’s scientific investigation. Hundreds of neuroscience papers have detailed how memory and personality are encoded structurally in synaptic connections, and recent advances in connectome imaging and brain simulation can be seen as a preview of the synthetic revival technologies to come.
Until now, the crucial unanswered questions were “How well does cryonics preserve the brain’s connectome?” and “Are there alternatives/modifications to cryonics that might preserve the connectome better and in a manner that could be demonstrated today?” The Brain Preservation Prize was put forward in 2010 to spur research that could definitively answer these questions. Now, five years later, these questions have been answered: Traditional cryonics procedures were not able to demonstrate (to the BPF’s satisfaction) preservation of the connectome, but the newly invented “Aldehyde-Stabilized Cryopreservation” technique was.
This result directly answers what has for decades been the main skeptical and scientific criticism against cryonics –that it does not provably preserve the delicate synaptic circuitry of the brain. As such, this research sets the stage for renewed interest within the scientific community, and offers a potential challenge to medical researchers to develop a human surgical procedure based on these successful animal experiments.
Abstract of Aldehyde-stabilized cryopreservation
We describe here a new cryobiological and neurobiological technique, aldehyde-stabilized cryopreservation (ASC), which demonstrates the relevance and utility of advanced cryopreservation science for the neurobiological research community. ASC is a new brain-banking technique designed to facilitate neuroanatomic research such as connectomics research, and has the unique ability to combine stable long term ice-free sample storage with excellent anatomical resolution. To demonstrate the feasibility of ASC, we perfuse-fixed rabbit and pig brains with a glutaraldehyde-based fixative, then slowly perfused increasing concentrations of ethylene glycol over several hours in a manner similar to techniques used for whole organ cryopreservation. Once 65% w/v ethylene glycol was reached, we vitrified brains at −135 °C for indefinite long-term storage. Vitrified brains were rewarmed and the cryoprotectant removed either by perfusion or gradual diffusion from brain slices. We evaluated ASC-processed brains by electron microscopy of multiple regions across the whole brain and by Focused Ion Beam Milling and Scanning Electron Microscopy (FIB-SEM) imaging of selected brain volumes. Preservation was uniformly excellent: processes were easily traceable and synapses were crisp in both species. Aldehyde-stabilized cryopreservation has many advantages over other brain-banking techniques: chemicals are delivered via perfusion, which enables easy scaling to brains of any size; vitrification ensures that the ultrastructure of the brain will not degrade even over very long storage times; and the cryoprotectant can be removed, yielding a perfusable aldehyde-preserved brain which is suitable for a wide variety of brain assays.