Implantable ‘stentrode’ to allow paralyzed patients to control an exoskeleton with their mind
February 10, 2016
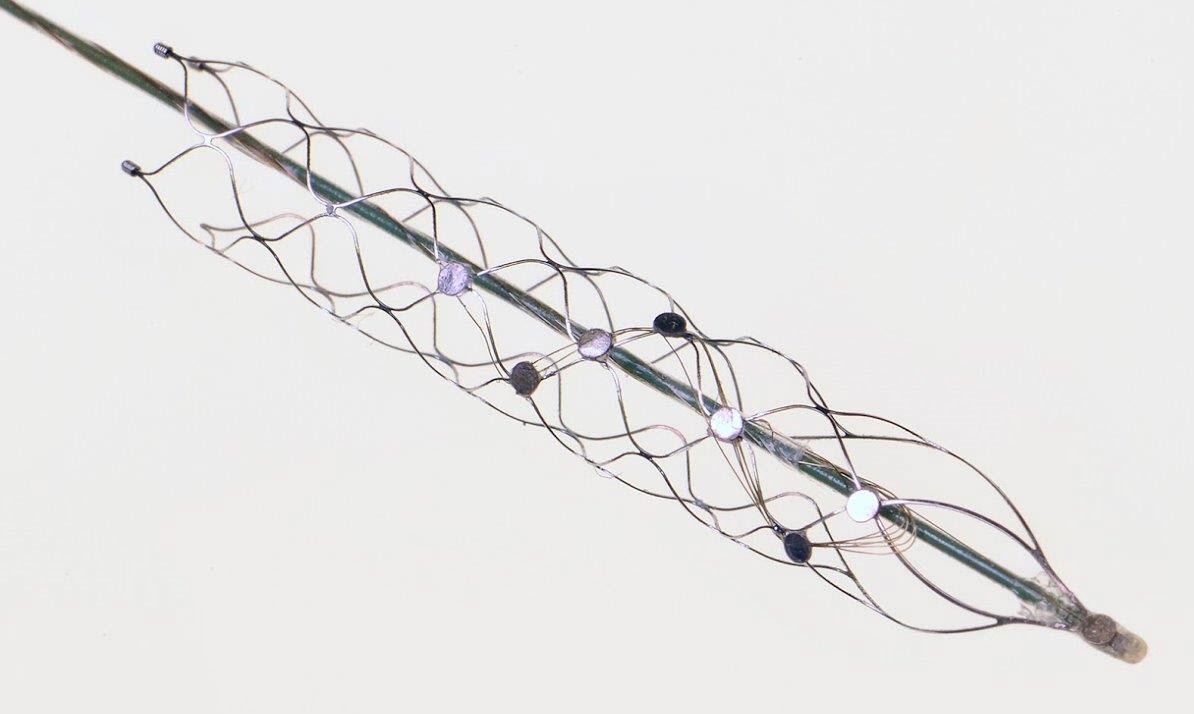
The “stentrode,” created by the University of Melbourne’s Vascular Bionics Laboratory, adapts an off-the-shelf self-expanding stent to include a recording electrode array. The device is delivered to the brain through blood vessels in the neck, thus avoiding many of the risks associated with traditional placement of neural implants through open-brain surgery. (credit: University of Melbourne)
A DARPA-funded research team has created a novel minimally invasive brain-machine interface and recording device that can be implanted into the brain through blood vessels, reducing the need for invasive surgery and the risks associated with breaching the blood-brain barrier when treating patients for physical disabilities and neurological disorders.
(credit: University of Melbourne)
The new technology, developed by University of Melbourne medical researchers under DARPA’s Reliable Neural-Interface Technology (RE-NET) program, promises to give people with spinal cord injuries new hope to walk again.
The brain-machine interface consists of a stent-based electrode (stentrode), which is implanted within a blood vessel next to the brain, and records the type of neural activity that has been shown in pre-clinical trials to move limbs through an exoskeleton or to control bionic limbs.
The new device is the size of a small paperclip and will be implanted in the first in-human trial at The Royal Melbourne Hospital in 2017.
The research results, published Monday Feb. 8 in Nature Biotechnology, show the device is capable of recording high-quality signals emitted from the brain’s motor cortex without the need for open brain surgery.
“We have been able to create the world’s only minimally invasive device that is implanted into a blood vessel in the brain via a simple day procedure, avoiding the need for high risk open brain surgery,” said Thomas Oxley, principal author and neurologist at The Royal Melbourne Hospital and Research Fellow at The Florey Institute of Neurosciences and the University of Melbourne.
Stroke and spinal cord injuries are leading causes of disability, affecting 1 in 50 people. There are 20,000 Australians with spinal cord injuries, with the typical patient a 19-year old male, and about 150,000 Australians left severely disabled after stroke.
The University of Melbourne | Stentrode in action
Implantable stent with electrodes
Co-principal investigator and biomedical engineer at the University of Melbourne, Nicholas Opie, said the concept was similar to an implantable cardiac pacemaker — electrical interaction with tissue using sensors inserted into a vein, but inside the brain.

Stentrode with 8 × 750 micrometer electrode discs (yellow arrow) self-expanding during deployment from catheter (green arrow). Scale bar, 3 mm. (credit: Thomas J. Oxley et al./Nature Biotechnology)
“The electrode array self-expands to stick to the inside wall of a vein, enabling the researchers to record local brain activity. By extracting the recorded neural signals, we can use these as commands to control wheelchairs, exoskeletons, prosthetic limbs or computers. In our first-in-human trial, that we anticipate will begin within two years, we are hoping to achieve direct brain control of an exoskeleton for three people with paralysis,” he said.
Thought control
“Currently, exoskeletons are controlled by manual manipulation of a joystick to switch between the various elements of walking — stand, start, stop, turn. The stentrode will be the first device that enables direct thought control of these devices.”
Professor Clive May, neurophysiologist at The Florey, said the data from the pre-clinical study highlighted that the implantation of the device was safe for long-term use. “Our study also showed that it was safe and effective to implant the device via angiography, which is minimally invasive compared with the high risks associated with open-brain surgery.
The authors note that “avoiding direct contact with cortical neurons may mitigate brain trauma and chronic local inflammation,” subject to additional evaluation.
The University of Melbourne | Stentrode: Moving with the power of thought
In addition to DARPA, the research was supported by Australia’s National Health and Medical Research Council, the U.S. Office of Naval Research Global, The Australian Defence Health Foundation, The Brain Foundation, and The Royal Melbourne Hospital Neuroscience Foundation.
Lighter, more agile exoskeleton helps the paralyzed to walk

Steven Sanchez, who was paralyzed from the waist down after a BMX accident, wears SuitX’s light, more agile Phoenix exoskeleton. (credit: SuitX)
Meanwhile, in related research (also based on initial funding from DARPA), SuitX, a spinoff of UC Berkeley’s Robotics and Human Engineering Laboratory robotics lab, introduced last week the Phoenix — a new lighter, more agile and lower-cost manually controlled exoskeleton.
The Phoenix is lightweight and has two motors at the hips and electrically controlled tension settings that tighten when the wearer is standing and swing freely when they’re walking. Users can control the movement of each leg and walk up to 1.1 miles per hour by pushing buttons integrated into a pair of crutches. It’s powered for up to eight hours by a battery pack worn in a backpack.
Developed from the Berkeley Lower Extremity Exoskeleton (BLEEX), the Phoenix is one of the lightest and most accessible exoskeletons available, according to SuitX. It can be adjusted to fit varied weights, heights, and leg sizes and can be used for a range of mobility hindrances. At $40,000, it’s about the half the cost of other exoskeletons that help restore mobility.
Abstract of Minimally invasive endovascular stent-electrode array for high-fidelity, chronic recordings of cortical neural activity
High-fidelity intracranial electrode arrays for recording and stimulating brain activity have facilitated major advances in the treatment of neurological conditions over the past decade. Traditional arrays require direct implantation into the brain via open craniotomy, which can lead to inflammatory tissue responses, necessitating development of minimally invasive approaches that avoid brain trauma. Here we demonstrate the feasibility of chronically recording brain activity from within a vein using a passive stent-electrode recording array (stentrode). We achieved implantation into a superficial cortical vein overlying the motor cortex via catheter angiography and demonstrate neural recordings in freely moving sheep for up to 190 d. Spectral content and bandwidth of vascular electrocorticography were comparable to those of recordings from epidural surface arrays. Venous internal lumen patency was maintained for the duration of implantation. Stentrodes may have wide ranging applications as a neural interface for treatment of a range of neurological conditions.