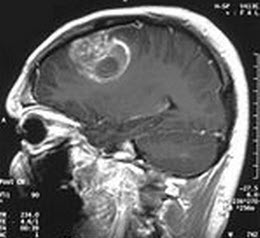
Scientists discover protein that can slow brain tumor growth in mice
May 14, 2014
Biochemists have identified a protein that can be used to slow down or speed up the growth of glioblastoma brain tumors in mice.
A preclinical study led by Eric J. Wagner, Ph.D., and Ann-Bin Shyu, Ph.D., of The University of Texas Health Science Center at Houston (UTHealth) and Wei Li, Ph.D., of Baylor College of Medicine appear in Nature.
“Our work could lead to the development of a novel therapeutic target that might slow down tumor progression,” said Wagner, assistant professor in the Department of Biochemistry and Molecular Biology at the UTHealth Medical School.
The research team discovered that reducing a protein called CFIm25 (which is critical to keeping Messenger RNA molecules* long in healthy cells) in a mouse model of brain cancer promotes tumor growth. They also found that restoring CFIm25 levels in brain tumors dramatically reduced their growth.
Additional preclinical tests are needed before the strategy can be evaluated in humans.
Brain and other nervous system cancers are expected to claim 14,320 lives in the United States this year.
Scientists at the The University of Texas Graduate School of Biomedical Sciences also contributed to the study, which received support from the Department of Defense, National Institutes of Health, the Houston Endowment, Inc., the Marnie Rose Foundation, and the William and Ella Owens Medical Research Foundation.
* Messenger RNA molecules use information from genes to make proteins, which create body tissues.
Abstract of Nature paper
The global shortening of messenger RNAs through alternative polyadenylation (APA) that occurs during enhanced cellular proliferation represents an important, yet poorly understood mechanism of regulated gene expression. The 3′ untranslated region (UTR) truncation of growth-promoting mRNA transcripts that relieves intrinsic microRNA- and AU-rich-element-mediated repression has been observed to correlate with cellular transformation; however, the importance to tumorigenicity of RNA 3′-end-processing factors that potentially govern APA is unknown. Here we identify CFIm25 as a broad repressor of proximal poly(A) site usage that, when depleted, increases cell proliferation. Applying a regression model on standard RNA-sequencing data for novel APA events, we identified at least 1,450 genes with shortened 3′ UTRs after CFIm25 knockdown, representing 11% of significantly expressed mRNAs in human cells. Marked increases in the expression of several known oncogenes, including cyclin D1, are observed as a consequence of CFIm25 depletion. Importantly, we identified a subset of CFIm25-regulated APA genes with shortened 3′ UTRs in glioblastoma tumours that have reduced CFIm25 expression. Downregulation of CFIm25 expression in glioblastoma cells enhances their tumorigenic properties and increases tumour size, whereas CFIm25 overexpression reduces these properties and inhibits tumour growth. These findings identify a pivotal role of CFIm25 in governing APA and reveal a previously unknown connection between CFIm25 and glioblastoma tumorigenicity.