Scientists find key protein for spinal cord repair in zebrafish
November 3, 2016
Duke University | Spinal Cord Injury and Regeneration in Zebrafish
Duke University scientists have found a protein that’s important for the ability of the freshwater zebrafish’s spinal cord to heal completely after being severed. Their study, published Nov. 4 in the journal Science, could generate new leads for what is a paralyzing and often fatal injury for humans.
Searching for the repair molecules
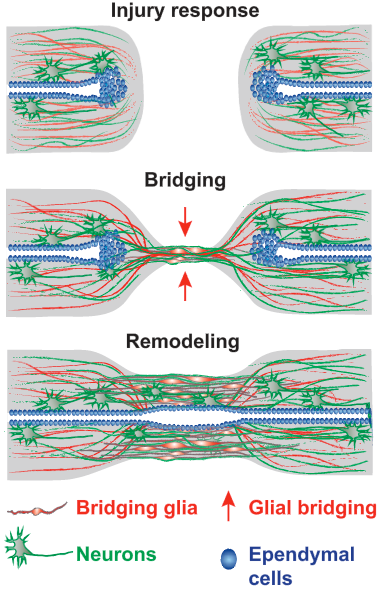
Schematic of the multistep process of spinal cord regeneration in zebrafish. (Injury response:) When the zebrafish’s severed spinal cord undergoes regeneration, a bridge forms. (Bridging:) The first cells extend projections into a distance tens of times their own length and connect across a wide gulf of the injury. Nerve cells follow. (Remodeling:) By 8 weeks, new nerve tissue has filled the gap and the animals have fully reversed their severe paralysis. (credit: Mayssa H. Mokalled et al./Science)
To understand what molecules were potentially responsible for this remarkable process, the scientists searched for the genes whose activity abruptly changed after spinal cord injury. Of dozens of genes strongly activated by injury, seven coded for proteins that are secreted from cells. One of these, called CTGF (connective tissue growth factor), was intriguing because its levels rose in the supporting cells, or glia, that formed the bridge in the first two weeks following injury.
What’s more, when they tried deleting CTGF genetically, those fish failed to regenerate.
The human CTGF protein is 87% similar in its amino acid building blocks to the zebrafish form. So when the team added the human version of CTGF to the injury site in fish, it boosted regeneration and the fish swam better by two weeks after the injury.
The second half of the CTGF protein seems to be the key to the healing, the group found. It’s a large protein, made of four smaller parts, and it has more than one function. That might make it easier to deliver and more specific as a therapy for spinal injuries.
Mouse studies next
Unfortunately, CTGF is probably not sufficient on its own for people to regenerate their own spinal cords, according to the study’s senior investigator, Kenneth Poss, professor of cell biology and director of the Regeneration Next initiative at Duke. Healing is more complex in mammals, in part because scar tissue forms around the injury. The Poss team expects studies of CTGF to move into mammals like mice to determine when they express CTGF, and in what cell types.
These experiments may reveal some answers to why zebrafish can regenerate whereas mammals cannot. It may be a matter of how the protein is controlled rather than its make-up, Poss said.
The group also plans to follow up on other proteins secreted after injury that were identified in their initial search, which may provide additional hints into the zebrafish’s secrets of regeneration.
Scientists at the Max Planck Institute for Heart and Lung Research were also involved in the research, which was supported by the National Institutes of Health, the Max Planck Society, and Duke University School of Medicine.
Abstract of Injury-induced ctgfa directs glial bridging and spinal cord regeneration in zebrafish
Unlike mammals, zebrafish efficiently regenerate functional nervous system tissue after major spinal cord injury.Whereas glial scarring presents a roadblock for mammalian spinal cord repair, glial cells in zebrafish form a bridge across severed spinal cord tissue and facilitate regeneration. We performed a genome-wide profiling screen for secreted factors that are up-regulated during zebrafish spinal cord regeneration. We found that connective tissue growth factor a (ctgfa) is induced in and around glial cells that participate in initial bridging events. Mutations in ctgfa disrupted spinal cord repair, and transgenic ctgfa overexpression and local delivery of human CTGF recombinant protein accelerated bridging and functional regeneration. Our study reveals that CTGF is necessary and sufficient to stimulate glial bridging and natural spinal cord regeneration.