Stomach-acid-powered micromotors tested in living animal
January 28, 2015
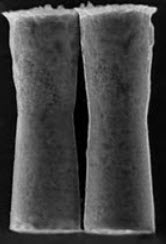
SEM image of Zinc micromotors (credit: UCSD)
Imagine a micromotor fueled by stomach acid that can take a bubble-powered ride inside a mouse — and that could one day be a safer, more efficient way to deliver drugs or diagnose tumors for humans.
That’s the goal of a team of researchers at the University of California, San Diego.
The experiment is the first to show that these micromotors can operate safely in a living animal, said Professors Joseph Wang and Liangfang Zhang of the NanoEngineering Department at the UC San Diego Jacobs School of Engineering.
Wang, Zhang and others have experimented with different designs and fuel systems for micromotors that can travel in water, blood and other body fluids in the lab.
“But this is the first example of loading and releasing a cargo in vivo,” said Wang. “We thought it was the logical extension of the work we have done, to see if these motors might be able to swim in stomach acid.”
How it works
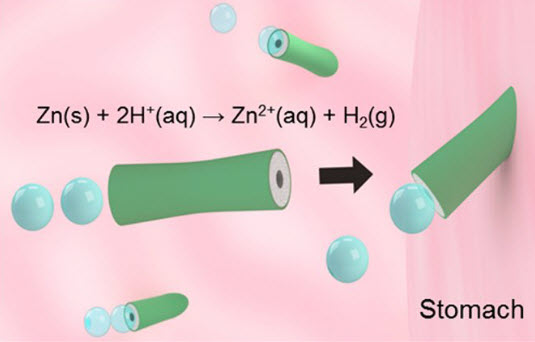
Schematic of the in vivo propulsion and tissue penetration of the zinc-based micromotors in mouse stomach (credit: Wei Gao et al./ACS Nano)
In the experiment, the mice ingested tiny drops of solution containing hundreds of the micromotors, which are 20 micrometers long. The motors become active as soon as they hit the stomach acid and zoom toward the stomach lining at a speed of 60 micrometers per second. They can self-propel like this for up to 10 minutes.
This propulsive burst improved how well the cone-shaped motors were able to penetrate and stick in the mucous layer covering the stomach wall, explained Zhang. “It’s the motor that can punch into this viscous layer and stay there, which is an advantage over more passive delivery systems,” he said.
The researchers found that nearly four times as many zinc micromotors found their way into the stomach lining compared with platinum-based micromotors, which don’t react with and can’t be fueled by stomach acid.
Wang said it may be possible to add navigation capabilities and other functions to the motors, to increase their targeting potential. Now that his team has demonstrated that the motors work in living animals, he noted, similar nanomachines soon may find a variety of applications including drug delivery, diagnostics, nanosurgery and biopsies of hard-to-reach tumors.
But is it safe?
The researchers explain that stomach acid reacts with the zinc body of the motors to generate a stream of hydrogen microbubbles that propel the motors forward. In their open-access study published in the journal ACS Nano, the researchers report that the motors lodged themselves firmly in the stomach lining of mice. As the zinc motors are dissolved by the acid, they disappear within a few days leaving no toxic chemical traces.
When they loaded up the motors with a test “payload” of gold nanoparticles, Wang, Zhang and their coworkers found that more of these particles reached the stomach lining when carried by the motors, compared to when the particles alone were swallowed. The motors delivered 168 nanograms of gold per gram of stomach tissue, compared to the 53.6 nanograms per gram that was delivered through the traditional oral route in the test.
“This initial work verifies that this motor can function in a real animal and is safe to use,” said Zhang.
JacobsSchoolNews | Stomach Acid-Powered Micromotors Get Their First Test in a Living Animal
Abstract of Artificial micromotors in the mouse’s stomach: A step toward in vivo use of synthetic motors
Artificial micromotors, operating on locally supplied fuels and performing complex tasks, offer great potential for diverse biomedical applications, including autonomous delivery and release of therapeutic payloads and cell manipulation. Various types of synthetic motors, utilizing different propulsion mechanisms, have been fabricated to operate in biological matrices. However, the performance of these man-made motors has been tested exclusively under in vitro conditions (outside the body); their behavior and functionalities in an in vivo environment (inside the body) remain unknown. Herein, we report an in vivo study of artificial micromotors in a living organism using a mouse model. Such in vivo evaluation examines the distribution, retention, cargo delivery, and acute toxicity profile of synthetic motors in mouse stomach via oral administration. Using zinc-based micromotors as a model, we demonstrate that the acid-driven propulsion in the stomach effectively enhances the binding and retention of the motors as well as of cargo payloads on the stomach wall. The body of the motors gradually dissolves in the gastric acid, autonomously releasing their carried payloads, leaving nothing toxic behind. This work is anticipated to significantly advance the emerging field of nano/micromotors and to open the door to in vivo evaluation and clinical applications of these synthetic motors.