The case of the silent synapses: Why are only 20% of synapses active during neurotransmission?
February 26, 2016
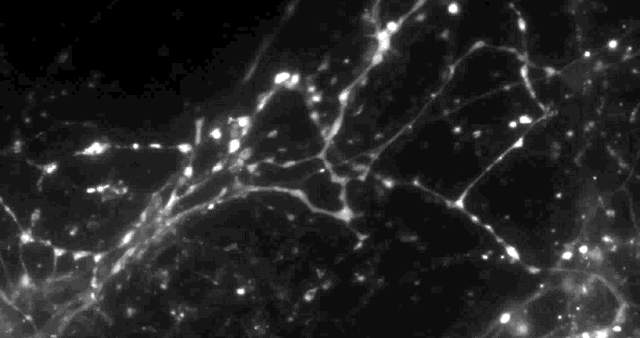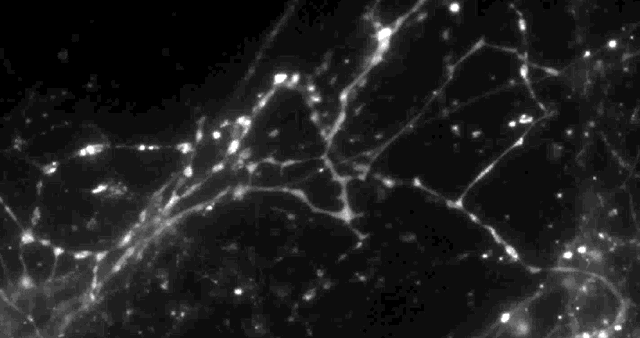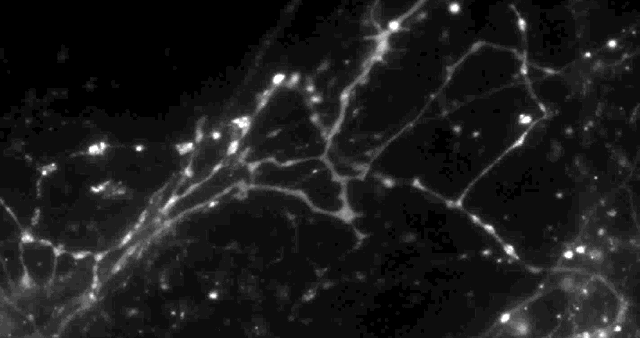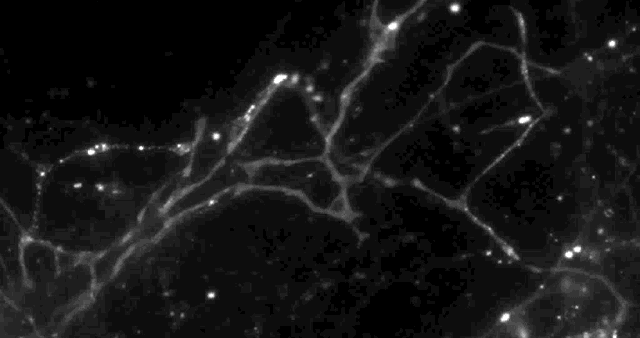
Using a fluorescent molecule to track neurotransmission of dopamine in mouse synapses, scientists made a puzzling discovery. … (credit: Sulzer Lab/Columbia University Medical Center)
Columbia University scientists recently tested a new optical technique to study how information is transmitted in the brains of mice and made a surprising discovery: When stimulated electrically to release dopamine (a neurotransmitter or chemical released by neurons, or nerve cells, to send signals to other nerve cells), only about 20 percent of synapses — the connections between cells that control brain activity — were active at any given time.
The effect had never been noticed. “Older techniques only revealed what was going on in large groups of synapses,” explained David Sulzer, PhD, professor of neurobiology in Psychiatry, Neurology, and Pharmacology at Columbia University Medical Center (CUMC). “We needed a way to observe the neurotransmitter activity of individual synapses, to help us better understand their intricate behavior.”
So Sulzer’s team turned to Dalibor Sames, PhD, associate professor of chemistry at Columbia, to develop a novel compound called “fluorescent false neurotransmitter 200” (FFN200). When added to brain tissue or nerve cells from mice, FFN200 mimicked the brain’s natural neurotransmitters, allowing the researchers to spy on chemical messaging in action, focusing on complex tasks such as learning and memory.
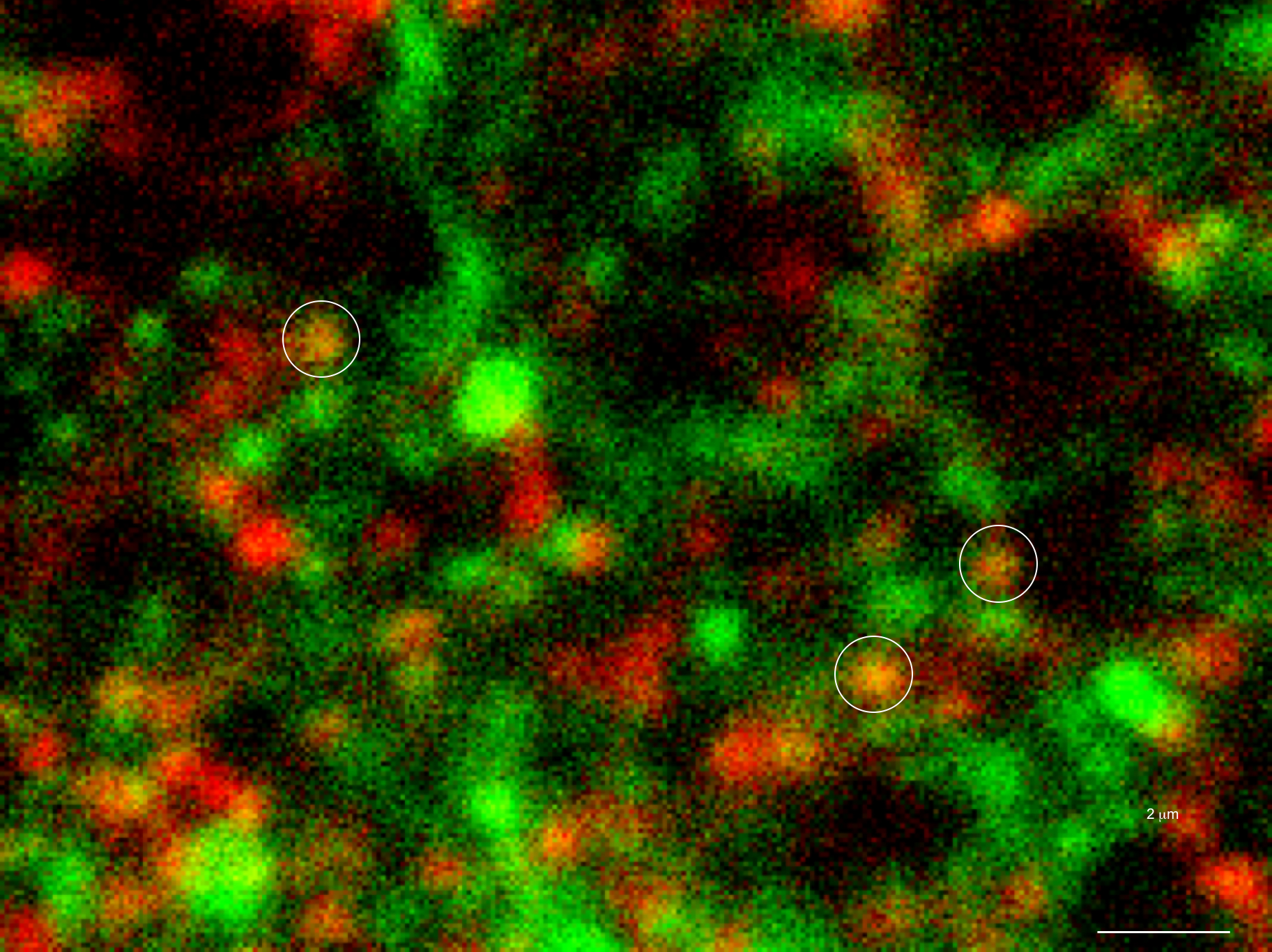
Only 20% of synapses (red) were observed to transmit dopamine. The rest (green) were found to be silent. (credit: Sulzer Lab/Columbia University Medical Center)
Silent synapses: unknown information coding?
Using a fluorescence microscope, the researchers were able for the first time to view the release and re-uptake of dopamine — a neurotransmitter involved in motor learning, habit formation, and reward-seeking behavior — in individual synapses.
When all the neurons were electrically stimulated in a sample of brain tissue, the researchers expected all the synapses to release dopamine. Instead, they found that less than 20 percent of dopaminergic synapses were active following a pulse of electricity.
One possibility: these silent synapses hint at a mechanism of information coding in the brain that’s yet to be revealed, the researchers hypothesize.
The study’s authors plan to pursue that hypothesis in future experiments and examine how other neurotransmitters behave. “If we can work this out, we may learn a lot more about how alterations in dopamine levels are involved in brain disorders such as Parkinson’s disease, addiction, and schizophrenia,” Sulzer said.
The study was published in the latest issue of Nature Neuroscience.
The authors note in the paper that “the state of silent vesicle clusters may be important in disorders such as schizophrenia, which show striatal hyperdopaminergia [excessive release of dopamine in the brain’s reward center] and cortical hypodopaminergia [low amounts of dopamine in the cortex] and processes of ‘unsilencing’ may have clinical applications for diseases such as Parkinson’s disease.”
Columbia Medical | Study Finds Only a Small Portion of Synapses May Be Active During Neurotransmission
Abstract of Fluorescent false neurotransmitter reveals functionally silent dopamine vesicle clusters in the striatum
Neurotransmission at dopaminergic synapses has been studied with techniques that provide high temporal resolution, but cannot resolve individual synapses. To elucidate the spatial dynamics and heterogeneity of individual dopamine boutons, we developed fluorescent false neurotransmitter 200 (FFN200), a vesicular monoamine transporter 2 (VMAT2) substrate that selectively traces monoamine exocytosis in both neuronal cell culture and brain tissue. By monitoring electrically evoked Ca2+ transients with GCaMP3 and FFN200 release simultaneously, we found that only a small fraction of dopamine boutons that exhibited Ca2+ influx engaged in exocytosis, a result confirmed with activity-dependent loading of the endocytic probe FM1-43. Thus, only a low fraction of striatal dopamine axonal sites with uptake-competent VMAT2 vesicles are capable of transmitter release. This is consistent with the presence of functionally ‘silent’ dopamine vesicle clusters and represents, to the best of our knowledge, the first report suggestive of presynaptically silent neuromodulatory synapses.