Targeting tumors using silver nanoparticles
June 18, 2014
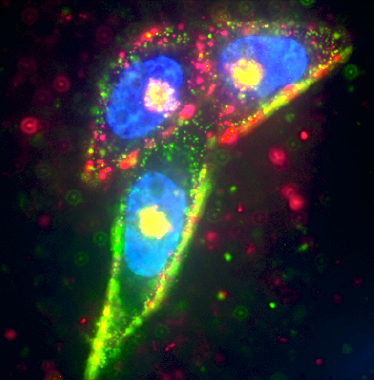
Prostate cancer cells were targeted by two separate silver nanoparticles (red and green), while the cell nucleus was labeled in blue using Hoescht dye (credit: UCSB)
Scientists at UC Santa Barbara have designed a silver spherical nanoparticle encased in a shell coated with a peptide that enables it to target tumor cells.
The shell is etchable so those nanoparticles that don’t hit their target can be broken down and eliminated. The research findings appear in the journal Nature Materials.
The core of the nanoparticle employs a phenomenon called plasmonics. In plasmonics, nanostructured metals such as gold and silver resonate in light and concentrate the electromagnetic field near the surface.
In this way, fluorescent dyes (to identify tumor cells) are enhanced, appearing about tenfold brighter than their natural state when no metal is present. When the core is etched, the enhancement goes away and the particle becomes dim.
UCSB’s Ruoslahti Research Laboratory also developed a simple etching technique using biocompatible chemicals to rapidly disassemble and remove the silver nanoparticles outside living cells. This method leaves only the intact nanoparticles for imaging or quantification, thus revealing which cells have been targeted and how much each cell internalized.
“The disassembly is an interesting concept for creating drugs that respond to a certain stimulus,” said Gary Braun, a postdoctoral associate in the Ruoslahti Lab in the Department of Molecular, Cellular and Developmental Biology (MCDB) and at Sanford-Burnham Medical Research Institute. “It also minimizes the off-target toxicity by breaking down the excess nanoparticles so they can then be cleared through the kidneys.”
Better penetration of target cells
This method for removing nanoparticles unable to penetrate target cells is unique. “By focusing on the nanoparticles that actually got into cells,” Braun said, “we can then understand which cells were targeted and study the tissue transport pathways in more detail.”
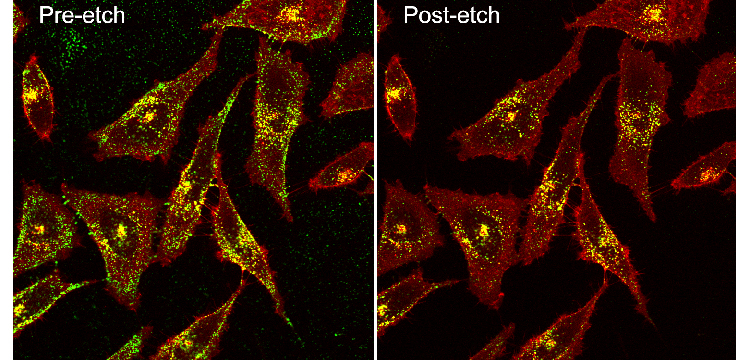
Pre and post-etch Confocal Prostate cancer cells with Green Silver RPARPAR_small (credit: Gary Braun)
Some drugs are able to pass through the cell membrane on their own, but many drugs, especially RNA and DNA genetic drugs, are charged molecules that are blocked by the membrane. These drugs must be taken in through endocytosis, the process by which cells absorb molecules by engulfing them.
“This typically requires a nanoparticle carrier to protect the drug and carry it into the cell,” Braun said. “And that’s what we measured: the internalization of a carrier via endocytosis.”
Because the nanoparticle has a core shell structure, the researchers can vary its exterior coating and compare the efficiency of tumor targeting and internalization. Switching out the surface agent enables the targeting of different diseases — or organisms in the case of bacteria — through the use of different target receptors. According to Braun, this should turn into a way to optimize drug delivery where the core is a drug-containing vehicle.
“These new nanoparticles have some remarkable properties that have already proven useful as a tool in our work that relates to targeted drug delivery into tumors,” said Erkki Ruoslahti, adjunct distinguished professor in UCSB’s Center for Nanomedicine and MCDB department and at Sanford-Burnham Medical Research Institute.
“They also have potential applications in combating infections. Dangerous infections caused by bacteria that are resistant to all antibiotics are getting more common, and new approaches to deal with this problem are desperately needed. Silver is a locally used antibacterial agent and our targeting technology may make it possible to use silver nanoparticles in treating infections anywhere in the body.”
Filling the gap in nanoparticle drug delivery
The objective of the research is to “create internalization assays to optimize targeted nanomedicine and drug delivery, to study the biological pathways that transport material from the blood stream into tissues, including tumors,” Braun explained in an email to KurzweilAI. In addition, the study is designed “to create dissassembling nanosystems that reduce side effects and toxicity to off-target organs, and to create injectable sensor systems that know when they are inside or outside a target cell in the body, and can relay that information via imaging.
“This is a new method that fills a gap in information regarding drug delivery using nanoparticles. By disassembling, or etching, any nanoparticles that have not entered cells, the method allows the cellular internalization step for nanotherapeutics to be studied in greater detail, in living cells and within the body.
“Internalization into cells is critical for the coming wave of genetic delivery vehicles. Silver-peptide nanoparticles were engineered as a model vehicle for high specificity to tumor receptors and injected into the blood stream. Silver plasmonics gives a variety of options for imaging and the core is easily oxidized to disassemble the material to remove the signal.
“In our experiments we show how targeted silver could monitored entering cells in a tumor. The nanoparticles were injected and then after some time the etchant was injected, causing a disassembly of extracellular nanoparticles. The fraction of the dose that was inside cells in the tumor was quantified, and was found to increase with the etching delay time, revealing the internalization kinetics of tumor targeting nanoparticles for the first time.
“The innovation will first be used as a research tool to optimize targeted drug delivery and scan biopsies to find the most effective nanoparticle. In vivo sensors may also be developed using etchable nanosystems.”
Abstract of Nature Materials paper
There is considerable interest in using nanoparticles as labels or to deliver drugs and other bioactive compounds to cells in vitro and in vivo. Fluorescent imaging, commonly used to study internalization and subcellular localization of nanoparticles, does not allow unequivocal distinction between cell surface-bound and internalized particles, as there is no methodology to turn particles ‘off’. We have developed a simple technique to rapidly remove silver nanoparticles outside living cells, leaving only the internalized pool for imaging or quantification. The silver nanoparticle (AgNP) etching is based on the sensitivity of Ag to a hexacyanoferrate–thiosulphate redox-based destain solution. In demonstration of the technique we present a class of multicoloured plasmonic nanoprobes comprising dye-labelled AgNPs that are exceptionally bright and photostable, carry peptides as model targeting ligands, can be etched rapidly and with minimal toxicity in mice, and that show tumour uptake in vivo.