The brain-computer interface goes wireless
March 3, 2013
A team of neuroengineers at Brown University has developed a fully implantable and rechargeable wireless brain sensor capable of relaying real-time broadband signals from up to 100 neurons in freely moving subjects.
Several copies of the novel low-power device, described in the open-access Journal of Neural Engineering, have been performing well in animal models for more than year, a first in the brain-computer interface field.
Brain-computer interfaces could help people with severe paralysis control devices with their thoughts.
Neuroscientists can use such a device to observe, record, and analyze the signals emitted by scores of neurons in particular parts of the animal model’s brain.
Brain-computer interfaces (BCIs) are used to assess the feasibility of people with severe paralysis being able to move assistive devices like robotic arms or computer cursors by thinking about moving their arms and hands.
Previous BCIs have required wired systems (such as Braingate, also developed at Brown).
“This was conceived very much in concert with the larger BrainGate team, including neurosurgeons and neurologists giving us advice as to what were appropriate strategies for eventual clinical applications,” said Nurmikko, who is also affiliated with the Brown Institute for Brain Science.
Borton is now spearheading the development of a collaboration between EPFL and Brown to use a version of the device to study the role of the motor cortex in an animal model of Parkinson’s disease.
“This new wireless system addresses a major need for the next step in providing a practical brain-computer interface,” said neuroscientist John Donoghue, the Wriston Professor of Neuroscience at Brown University and director of the Brown Institute for Brain Science.
How the ‘brain radio’ works

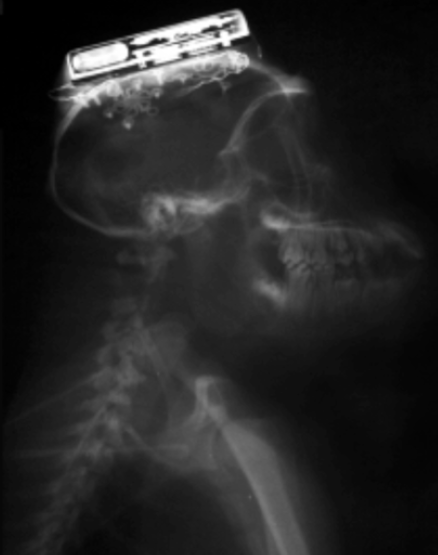
Hermetically sealed, wireless neural interface, ready for implanting under the skin (credit: David A Borton et al./J. Neural Eng.)
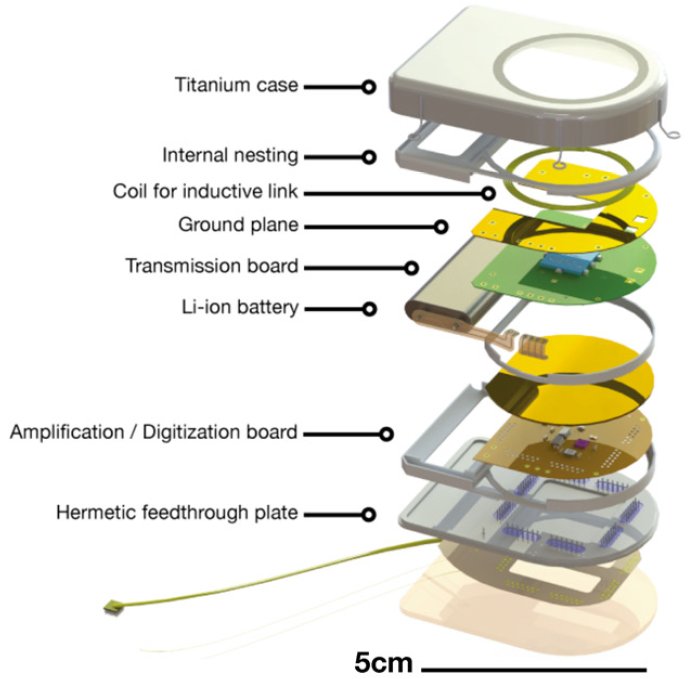
A pill-sized chip of electrodes implanted on the cortex sends signals through uniquely designed electrical connections into the device’s laser-welded, hermetically sealed 2.2 inches-long, 9 mm thick titanium “can.”
It houses an entire signal processing system: a lithium ion battery, ultralow-power integrated circuits for signal processing and conversion, wireless radio,infrared transmitters, and a copper coil for recharging.
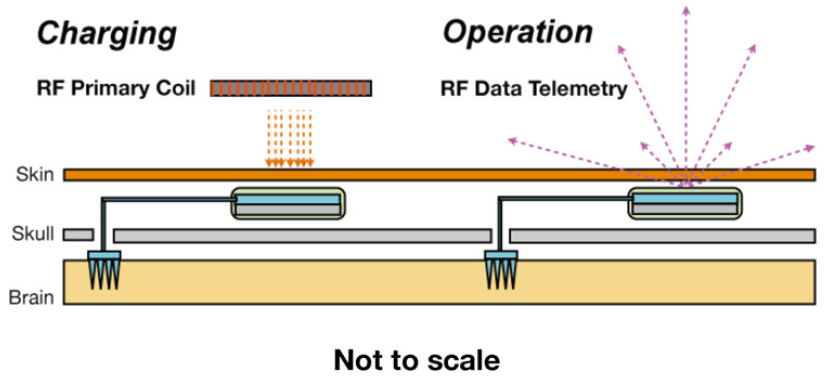
Wireless neural interface (implant), showing microelectrode arrays (inside the skull) for pickup up neural signals, and two boards in the implant for sending RF data for recording and for inductive battery-charging (from external RF primary coil) (credit: David A Borton et al./J. Neural Eng.)
“What makes the achievement discussed in this paper unique is that it’s the first fully implanted neural interface microsystem operated wirelessly; and how it integrated many individual innovations into a complete system,” said lead author David Borton, a former Brown graduate student and postdoctoral research associate who is now at Ecole Polytechnique Federale Lausanne in Switzerland..
“It has operated more than 12 months in large animal models — a milestone for potential [human] clinical translation.”
The device transmits data at 24 Mbps via 3.2 and 3.8 Ghz microwave frequencies (in between WiFi frequency bands) to an external receiver. After a two-hour charge, delivered wirelessly through the scalp via magnetic induction, it can operate for more than six hours.
“The device uses less than 100 milliwatts of power, a key figure of merit,” Nurmikko said.
Co-author Ming Yin, a Brown postdoctoral scholar and electrical engineer, said one of the major challenges that the team overcame in building the device was optimizing its performance to meet the requirements that the implant device be small, low-power, and leak-proof, potentially for decades.
Freeing subjects to move around in the real world
The team worked closely with neurosurgeons to implant the device in three pigs and three rhesus macaque monkeys. The research in these six animals has been helping scientists better observe complex neural signals for as long as 16 months so far. In the new paper, the team shows some of the rich neural signals they have been able to record in the lab. Ultimately this could translate to significant advances that can also inform human neuroscience.
Current wired systems constrain the actions of research subjects, said Arto Nurmikko, professor of engineering at Brown University, who oversaw the device’s invention. The value of wireless transmission is that it frees subjects to move however they intend, allowing them to produce a wider variety of more realistic behaviors.
If neuroscientists want to observe the brain signals produced during some running or foraging behaviors, for instance, they can’t use a cabled sensor to study how neural circuits would form those plans for action and execution or strategize in decision making.
In the experiments in the new paper, the device is connected to one array of 100 cortical electrodes, but the new device design allows for multiple arrays to be connected, Nurmikko said. That would allow scientists to observe ensembles of neurons in multiple related areas of a brain network.
The new wireless device is not approved for use in humans and is not used in clinical trials of brain-computer interfaces. It was designed, however, with that motivation.
The researchers are continuing work on advancing the device for even larger amounts of neural data transmission, reducing its size even further, and improving other aspects of the device’s safety and reliability so that it can someday be considered for clinical application in people with movement disabilities.
The device could also conceivably be used in the Brain Activity Map project.
Funding was provided by the National Institutes of Health/National Institute of Biomedical Imaging and Bioengineering, and National Institute of Neurological Disorders and Stroke, with partial support from the National Science Foundation and the Defense Advanced Research Projects Agency.