Will ‘borophene’ replace graphene as a better conductor of electrons?
February 5, 2014
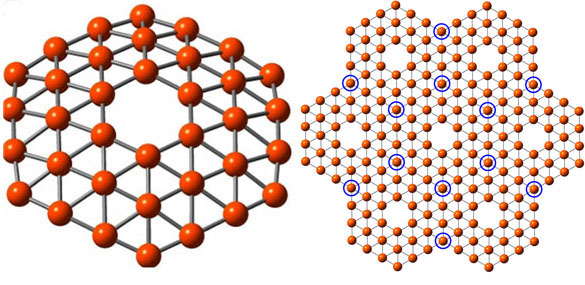
A 36-atom cluster of boron, left, arranged as a flat disc with a hexagonal hole in the middle, fits the theoretical requirements for making a one-atom-thick boron sheet, right, a theoretical nanomaterial dubbed “borophene” (credit: Wang lab/Brown University)
Researchers from Brown University have found evidence for a theoretical material they call “borophene” — a boron-based competitor to graphene.
Boron is carbon’s neighbor on the periodic table. Borophene has 36 boron atoms in a flat disc with a hexagonal hole in the middle.
Borophene is predicted to be fully metallic, whereas graphene is a semi-metal. That means borophene might end up being a better conductor than graphene, according to Lai-Sheng Wang, professor of chemistry at Brown.
Borophene can also potentially form two-dimensional sheets, similar to graphene. And Borophene’s boron-boron bond is also nearly as strong as the graphene’s carbon-carbon bond, said Wang.
The research involved a combination of laboratory experiments and computational modeling.
The researchers told KurzweilAI that a lot of work is still required to determine practical applications.
Abstract of Nature Communications paper
Boron is carbon’s neighbour in the periodic table and has similar valence orbitals. However, boron cannot form graphene-like structures with a honeycomb hexagonal framework because of its electron deficiency. Computational studies suggest that extended boron sheets with partially filled hexagonal holes are stable; however, there has been no experimental evidence for such atom-thin boron nanostructures. Here, we show experimentally and theoretically that B36 is a highly stable quasiplanar boron cluster with a central hexagonal hole, providing the first experimental evidence that single-atom layer boron sheets with hexagonal vacancies are potentially viable. Photoelectron spectroscopy of B36− reveals a relatively simple spectrum, suggesting a symmetric cluster. Global minimum searches for B36− lead to a quasiplanar structure with a central hexagonal hole. Neutral B36 is the smallest boron cluster to have sixfold symmetry and a perfect hexagonal vacancy, and it can be viewed as a potential basis for extended two-dimensional boron sheets.