With the ‘Bionanoprobe,’ rapid freezing leads to better nanoscale imaging of cells
February 28, 2014
Images of a frozen-hydrated algae cell. (a) Some cell ultrastructure is shown using differential phase contrast imaging. (b) Distributions of zinc, iron, and potassium are visible in this X-ray fluorescence image. (Credit: ANL)
For scientists to determine if a cell is functioning properly, they must destroy it (with X-rays), possibly giving false accounts of how the cell actually works.
Now, researchers at the U.S. Department of Energy’s (DOE) Argonne National Laboratory have created a new probe that freezes cells to “see” at greater detail without damaging the sample.*
Traditional X-ray fluorescence methods look at cells that have either been immersed in water or dehydrated. For wet specimens at room temperature, the radiation can break the bonds linking molecules together and cause them to scatter, changing the sample’s structure.
For dehydrated specimens, potassium and other diffusible ions are washed away during chemical fixation, which kills the cell and loosens the cell membrane, allowing ions to escape. Moreover, when the sample is dehydrated, the cell can shrink, distort or even collapse.
The Bionanoprobe
To address this issue, Argonne researchers developed a hard X-ray fluorescence nanoprobe called the Bionanoprobe, which makes three-dimensional images that map out the locations of trace elements, like iron or potassium, in frozen biological samples.
“We don’t want to dry the sample; we want to keep it hydrated,” says Chen. “We plunge the sample into liquid ethane at very high speeds and then look at the frozen sample directly.”
In a process reminiscent of cyronics, rapidly cooling biological specimens to temperatures of -260°F preserves the natural state of a cell’s organelles and trace elements while retaining the water in the sample. The Bionanoprobe’s vacuum chamber eliminates frosting and convective heating and automatically acquires tomographic (sectioned images) data sets.
The Bionanoprobe can also produce extremely high-resolution images at the smallest scales — below 100 nanometers. Si Chen, principal author of the study, uses X-ray optics called zone plates to focus the X-ray beam down to a miniscule small spot. A simple scan produces an image with a full fluorescent spectrum for each scanning step.
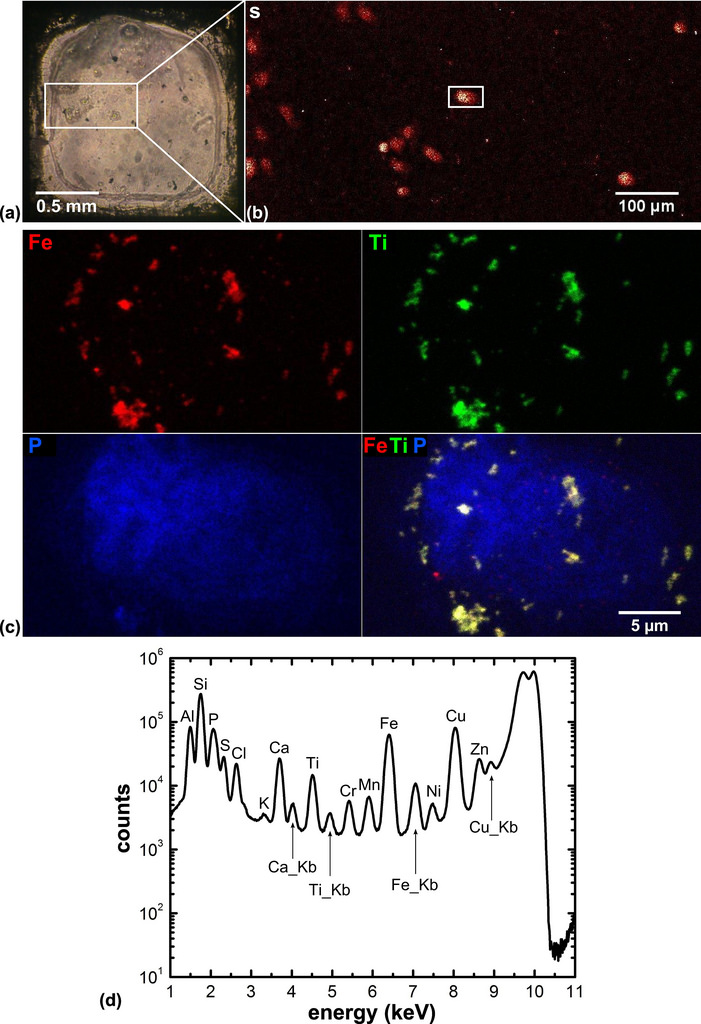
A recent study created X-ray fluorescence images of an immortal cervical cancer cell line called HeLa cells. The samples were plunge-frozen, chemically fixed and then treated with an iron oxide core in a titanium dioxide shell nanocomposite, which allowed researchers to determine if the nanocomposites actually made it into the cell nucleus.
Gale Woloschak, professor at Northwestern University’s Feinberg School of Medicine, who conducted the study, had created nanoparticles that target and kill cancer cells, but when the researchers wanted to see where the nanoparticles actually wound up in the cell, they ran into trouble with traditional X-ray methods.
“This is the problem,” says Woloschak. “If you think of how two-dimensional X-ray imaging works, X-rays penetrate through the entire cell, so it’s hard to determine whether the nanoparticles are above, below or inside the nucleus. What the Bionanoprobe does is give us a three-dimensional image — we could actually see that the nanoparticles were imbedded in the nucleus.”
The work is reported in “The Bionanoprobe: hard X-ray fluorescence nanoprobe with cryogenic capabilities,” published in the Journal of Synchrotron Radiation. Funding was provided by the National Institutes of Health.
* Two other Department of Energy labs, Pacific Northwest (PNNL) and Lawrence Livermore National Laboratories (LLNL), took another approach to the X-ray problem by using free-electron lasers to create images that accurately reflect the known structure of proteins (KurzweiAI news article here).
Abstract of Journal of Synchrotron Radiation paper
Hard X-ray fluorescence microscopy is one of the most sensitive techniques for performing trace elemental analysis of biological samples such as whole cells and tissues. Conventional sample preparation methods usually involve dehydration, which removes cellular water and may consequently cause structural collapse, or invasive processes such as embedding. Radiation-induced artifacts may also become an issue, particularly as the spatial resolution increases beyond the sub-micrometer scale. To allow imaging under hydrated conditions, close to the `natural state’, as well as to reduce structural radiation damage, the Bionanoprobe (BNP) has been developed, a hard X-ray fluorescence nanoprobe with cryogenic sample environment and cryo transfer capabilities, dedicated to studying trace elements in frozen-hydrated biological systems. The BNP is installed at an undulator beamline at sector 21 of the Advanced Photon Source. It provides a spatial resolution of 30 nm for two-dimensional fluorescence imaging. In this first demonstration the instrument design and motion control principles are described, the instrument performance is quantified, and the first results obtained with the BNP on frozen-hydrated whole cells are reported.