3-D printed ‘building blocks’ of life
November 4, 2015
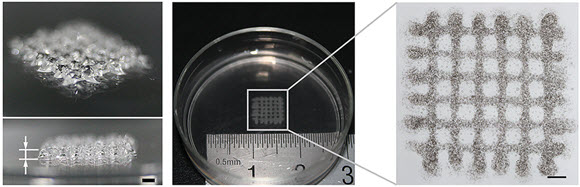
Images of printed embryonic stem cells, or embryoid bodies (credit: Liliang Ouyang et al./Biofabrication)
Chinese and U.S. scientists have developed a 3-D printing method capable of producing embryoid bodies — highly uniform “blocks” of embryonic stem cells. These cells, which are capable of generating all cell types in the body, could be used to build tissue structures and potentially even micro-organs.
The results were published Wednesday Nov. 4 in an open-access paper in the journal Biofabrication. “The embryoid body is uniform and homogenous, and serves as a much better starting point for further tissue growth,” explains Wei Sun, a lead author on the paper.
The researchers, based at Tsinghua University, Beijing, China, and Drexel University, Philadelphia, used extrusion-based 3-D printing to produce a grid-like 3-D structure to grow an embryoid body that demonstrated cell viability and rapid self-renewal for 7 days while maintaining high pluripotentcy.
“Two other common methods of printing these cells are two-dimensional (in a petri dish) or via the ‘suspension’ method [see ‘Better bioprinting with stem cells‘], where a ‘stalagmite’ of cells is built up by material being dropped via gravity,” said Sun. “However, these don’t show the same cell uniformity and homogenous proliferation. I think that we’ve produced a 3-D microenvironment that is much more like that found in vivo for growing embryoid bodies, which explains the higher levels of cell proliferation.”
The researchers hope that this technique can be developed to produce embryoid bodies at high throughput, providing the basic building blocks for other researchers to perform experiments on tissue regeneration and/or for drug screening studies.
The researchers say the next step is to find out more about how to vary the size of the embryoid body by changing the printing and structural parameters, and how varying the embryoid body size leads to “manufacture” of different cell types.
“In the longer term, we’d like to produce controlled heterogeneous embryonic bodies,” said Sun. “This would promote different cell types developing next to each other, which would lead the way for growing micro-organs from scratch within the lab.”
Abstract of Three-dimensional bioprinting of embryonic stem cells directs highly uniform embryoid body formation
With the ability to manipulate cells temporarily and spatially into three-dimensional (3D) tissue-like construct, 3D bioprinting technology was used in many studies to facilitate the recreation of complex cell niche and/or to better understand the regulation of stem cell proliferation and differentiation by cellular microenvironment factors. Embryonic stem cells (ESCs) have the capacity to differentiate into any specialized cell type of the animal body, generally via the formation of embryoid body (EB), which mimics the early stages of embryogenesis. In this study, extrusion-based 3D bioprinting technology was utilized for biofabricating ESCs into 3D cell-laden construct. The influence of 3D printing parameters on ESC viability, proliferation, maintenance of pluripotency and the rule of EB formation was systematically studied in this work. Results demonstrated that ESCs were successfully printed with hydrogel into 3D macroporous construct. Upon process optimization, about 90% ESCs remained alive after the process of bioprinting and cell-laden construct formation. ESCs continued proliferating into spheroid EBs in the hydrogel construct, while retaining the protein expression and gene expression of pluripotent markers, like octamer binding transcription factor 4, stage specific embryonic antigen 1 and Nanog. In this novel technology, EBs were formed through cell proliferation instead of aggregation, and the quantity of EBs was tuned by the initial cell density in the 3D bioprinting process. This study introduces the 3D bioprinting of ESCs into a 3D cell-laden hydrogel construct for the first time and showed the production of uniform, pluripotent, high-throughput and size-controllable EBs, which indicated strong potential in ESC large scale expansion, stem cell regulation and fabrication of tissue-like structure and drug screening studies.