3D-printing a new lifelike liver tissue for drug screening
February 15, 2016
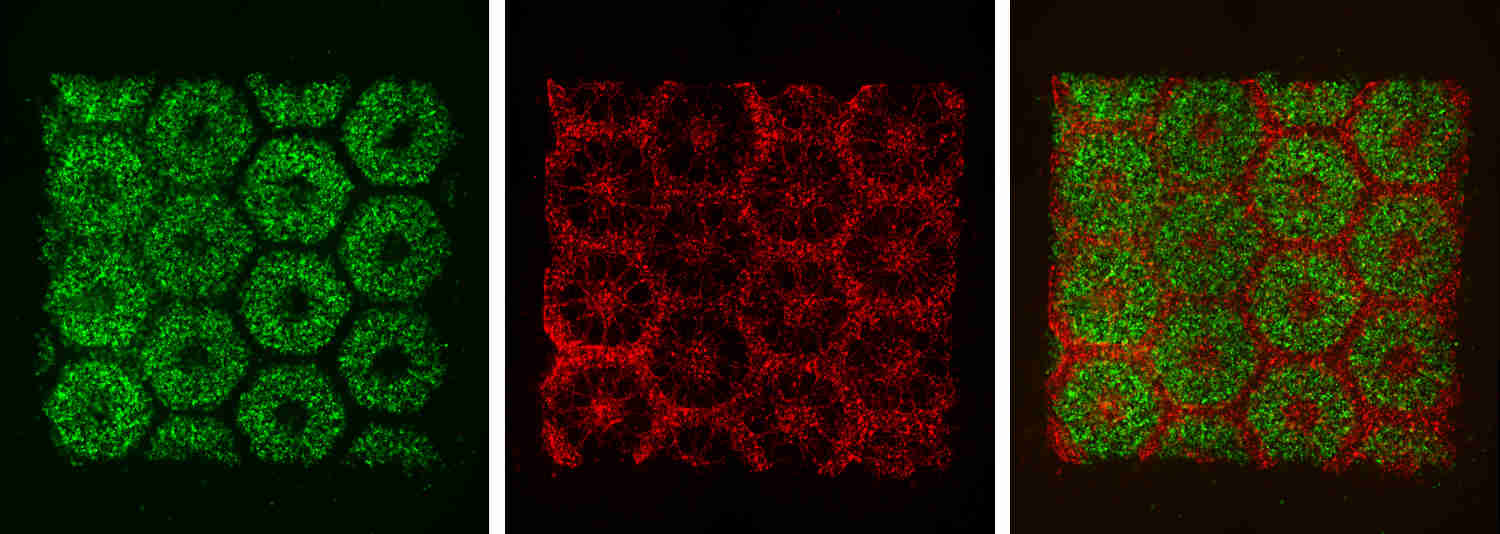
Images of the 3D-printed parts of the biomimetic liver tissue: liver cells derived from human induced pluripotent stem cells (left), endothelial and mesenchymal supporing cells (center), and the resulting organized combination of multiple cell types (right). (credit: Chen Laboratory, UC San Diego)
University of California, San Diego researchers have 3D-printed a tissue that closely mimics the human liver’s sophisticated structure and function. The new model could be used for patient-specific drug screening and disease modeling and could help pharmaceutical companies save time and money when developing new drugs, according to the researchers.
The liver plays a critical role in how the body metabolizes drugs and produces key proteins, so liver models are increasingly being developed in the lab as platforms for drug screening. However, so far, the models lack both the complex micro-architecture and diverse cell makeup of a real liver. For example, the liver receives a dual blood supply with different pressures and chemical constituents.
So the team employed a novel bioprinting technology that can rapidly produce complex 3D microstructures that mimic the sophisticated features found in biological tissues.
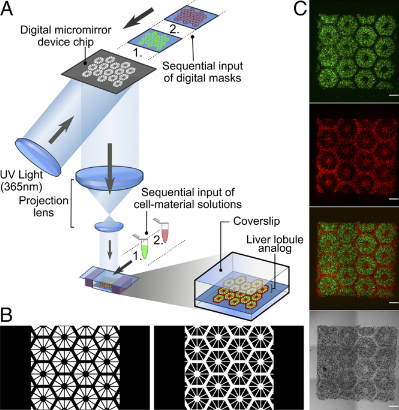
3D bioprinting of hydrogel-based hepatic (liver) construct. (A) Schematic diagram of a two-step 3D-bioprinting approach in which hiPSC-HPCs (human induced pluripotent stem cell-derived hepatic progenitor cells) were patterned by the first digital mask, followed by the patterning of supporting cells using a second digital mask. (B) Grayscale digital masks corresponding to polymerizing lobule structure (Left) and vascular structure (Right) designed for two-step bioprinting. The white patterns represent the light-reflecting patterns for photo-polymerization. (C) Images (5x) taken under fluorescent and bright field channels showing patterns of hiPSC-HPCs (green) and supporting cells (red). (credit: Xuanyi Ma et al./PNAS)
The liver tissue was printed in two steps.
- The team printed a honeycomb pattern of 900-micrometer-sized hexagons, each containing liver cells derived from human induced pluripotent stem cells. An advantage of human induced pluripotent stem cells is that they are patient-specific, which makes them ideal materials for building patient-specific drug screening platforms. And since these cells are derived from a patient’s own skin cells, researchers don’t need to extract any cells from the liver to build liver tissue.
- Then, endothelial and mesenchymal supporting cells were printed in the spaces between the stem-cell-containing hexagons.
The entire structure — a 3 × 3 millimeter square, 200 micrometers thick — takes just seconds to print. The researchers say this is a vast improvement over other methods to print liver models, which typically take hours. Their printed model was able to maintain essential functions over a longer time period than other liver models. It also expressed a relatively higher level of a key enzyme that’s considered to be involved in metabolizing many of the drugs administered to patients.
“It typically takes about 12 years and $1.8 billion to produce one FDA-approved drug,” said Shaochen Chen, NanoEngineering professor at the UC San Diego Jacobs School of Engineering. “That’s because over 90 percent of drugs don’t pass animal tests or human clinical trials. We’ve made a tool that pharmaceutical companies could use to do pilot studies on their new drugs, and they won’t have to wait until animal or human trials to test a drug’s safety and efficacy on patients. This would let them focus on the most promising drug candidates earlier on in the process.”
The work was published the week of Feb. 8 in the online early edition of Proceedings of the National Academy of Sciences.
Abstract of Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting
The functional maturation and preservation of hepatic cells derived from human induced pluripotent stem cells (hiPSCs) are essential to personalized in vitro drug screening and disease study. Major liver functions are tightly linked to the 3D assembly of hepatocytes, with the supporting cell types from both endodermal and mesodermal origins in a hexagonal lobule unit. Although there are many reports on functional 2D cell differentiation, few studies have demonstrated the in vitro maturation of hiPSC-derived hepatic progenitor cells (hiPSC-HPCs) in a 3D environment that depicts the physiologically relevant cell combination and microarchitecture. The application of rapid, digital 3D bioprinting to tissue engineering has allowed 3D patterning of multiple cell types in a predefined biomimetic manner. Here we present a 3D hydrogel-based triculture model that embeds hiPSC-HPCs with human umbilical vein endothelial cells and adipose-derived stem cells in a microscale hexagonal architecture. In comparison with 2D monolayer culture and a 3D HPC-only model, our 3D triculture model shows both phenotypic and functional enhancements in the hiPSC-HPCs over weeks of in vitro culture. Specifically, we find improved morphological organization, higher liver-specific gene expression levels, increased metabolic product secretion, and enhanced cytochrome P450 induction. The application of bioprinting technology in tissue engineering enables the development of a 3D biomimetic liver model that recapitulates the native liver module architecture and could be used for various applications such as early drug screening and disease modeling.