A ‘3D printer’ for customized small molecules such as drugs
March 12, 2015
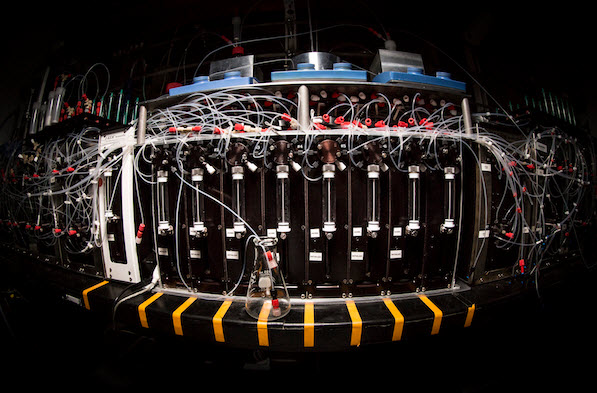
A machine in University of Illinois chemistry professor Martin Burke’s lab assembles complex small molecules out of simple chemical building blocks, like a 3D printer on the molecular level (credit: L. Brian Stauffer)
Howard Hughes Medical Institute scientists have developed a simpler way to synthesize small molecules, eliminating a major bottleneck in creating new medicines.
As the scientists note in the March 13, 2015, issue of the journal Science, “small-molecule syntheses typically employ strategies and purification methods that are highly customized for each target, thus requiring automation solutions to be developed [inefficiently] on an ad hoc basis.”
According to Martin Burke, an HHMI early career scientist at the University of Illinois at Urbana-Champaign who led the research, the highly customized approach that chemists have long relied on to synthesize small molecules is time consuming and inaccessible to most researchers.
“A lot of great medicines have not been discovered yet because of this synthesis bottleneck,” he says.
“The problem is, [small molecules] are really hard to make. Even though they’re small, they’re very complex, and it usually takes a highly trained specialist and long time to figure out each one,” Burke noted in a video (see below).
A building block strategy
So they explored the question: Could small molecules be prepared using a common building block strategy, which is currently done with large molecules (such as peptides)?
To do that, they developed a single automated process to synthesize 14 distinct classes of small molecules from a common set of building blocks using a single machine, which they describe as a “3D printer” for small molecules.
Burke’s team envisions expanding the approach to enable the production of thousands of potentially useful molecules. “The vision is that anybody could go to a website, pick the building blocks they want, instruct their assembly through the web, and the small molecules would get synthesized and shipped,” Burke says.
“Achieving this goal could do for chemistry what 3D printing did for engineering: make it fast, flexible, and accessible to everyone. We’re not there yet, but we now have an actionable roadmap toward on-demand small-molecule synthesis for non-specialists.”
UIUC | A Molecule-Making Machine
Nature produces an abundance of small molecules, and scientists have already adapted many of them for practical applications. The vast majority of drugs are considered small molecules, as are many important biological research tools. A wide-range of technologies, including LEDs, diagnostic tools, and solar cells also rely on small molecules. “Small molecules have already had a big impact on the world,” says Burke. “But we’ve barely touched the surface of what they’re capable of achieving. In large part, that’s because there’s a major synthesis bottleneck that precludes accessing all of their functional potential.”
Burke explains that chemists almost always develop a customized approach for manufacturing small molecules, designing a series of chemical reactions that, when applied to the right starting materials, yield the desired product. “Every time you make a molecule you have to develop a unique strategy. That customization is slow,” he says. Furthermore, it requires expertise. “Currently you have to have a high degree of training in synthesis to make small molecules,” Burke says.
In his research, Burke has been exploring the potential of small molecules to treat disease. Plants, animals, and microbes manufacture many small molecules with protein-like functions, and with some precise chemical modifications, Burke suspects it may be possible to optimize some of these natural products to mimic the function of missing proteins enough to restore patients’ health. To do that, he says, his team needs to synthesize and test not just the small molecule found in nature, but also new versions with targeted modifications.
Making those molecules is a major barrier to drug discovery, Burke says. “Doing real atomistic modifications to transform nature’s starting points into actual medicines is really, really challenging. The slow step in most cases in the synthesis. As a result, many natural products don’t get worked on in any practical way.”
Burke’s team took cues from nature to streamline the synthesis of the molecules they were studying, developing an approach that they have now expanded to make more general. “Nature makes most small molecules the same way,” Burke says. “There are a small number of building blocks that are coupled together over and over again, using the same kind of chemistry in an iterative fashion.” That means small molecules are inherently modular. So when Burke’s team analyzed the chemical structures of thousands of different natural products, patterns emerged. “There are building blocks that appear over and over again, and we’ve been able to dissect out the building blocks that are most common,” he says.
The small-molecule synthesizer that Burke’s team built takes these building blocks – each with two chemical connectors that can be readily linked to the corresponding part on another building block — and snaps them together like pop beads using a standard chemical reaction. The team used the approach to synthesize 14 different small molecules, ranging from relatively straightforward linear structures to densely folded molecules featuring several chemical rings.
Burke’s team has developed hundreds of these chemical building blocks and made them commercially available. “But it’s not really about the numbers,” he says. “We are showing that with a very reasonable number of building blocks we can make many different types of natural products.”
Burke says the technology is ready now to synthesize a range of very complex natural products, meaning the atom-by-atom modifications that researchers need to optimize these molecules into therapeutic compounds or technological tools are now accessible. He has founded a company, REVOLUTION Medicines, to use and continue to develop the technology for this purpose.
Ultimately, Burke says, he is excited about the opportunity to empower non-specialists – all kinds of scientists, engineers, medical doctors, and even the public – to produce small molecules. “When you put the power to manufacture into the hands of everyone, history speaks toward tremendous impact,” he says. “A 3D printer for molecules could allow us to harness all the creativity, innovation, and outside-the-box thinking that comes when non-experts start to use technology that used to only be in the hands of a select few.”
Abstract of Synthesis of many different types of organic small molecules using one automated process
Small-molecule synthesis usually relies on procedures that are highly customized for each target. A broadly applicable automated process could greatly increase the accessibility of this class of compounds to enable investigations of their practical potential. Here we report the synthesis of 14 distinct classes of small molecules using the same fully automated process. This was achieved by strategically expanding the scope of a building block–based synthesis platform to include even Csp3-rich polycyclic natural product frameworks and discovering a catch-and-release chromatographic purification protocol applicable to all of the corresponding intermediates. With thousands of compatible building blocks already commercially available, many small molecules are now accessible with this platform. More broadly, these findings illuminate an actionable roadmap to a more general and automated approach for small-molecule synthesis.