A breakthrough new method for 3D-printing living tissues
August 21, 2017
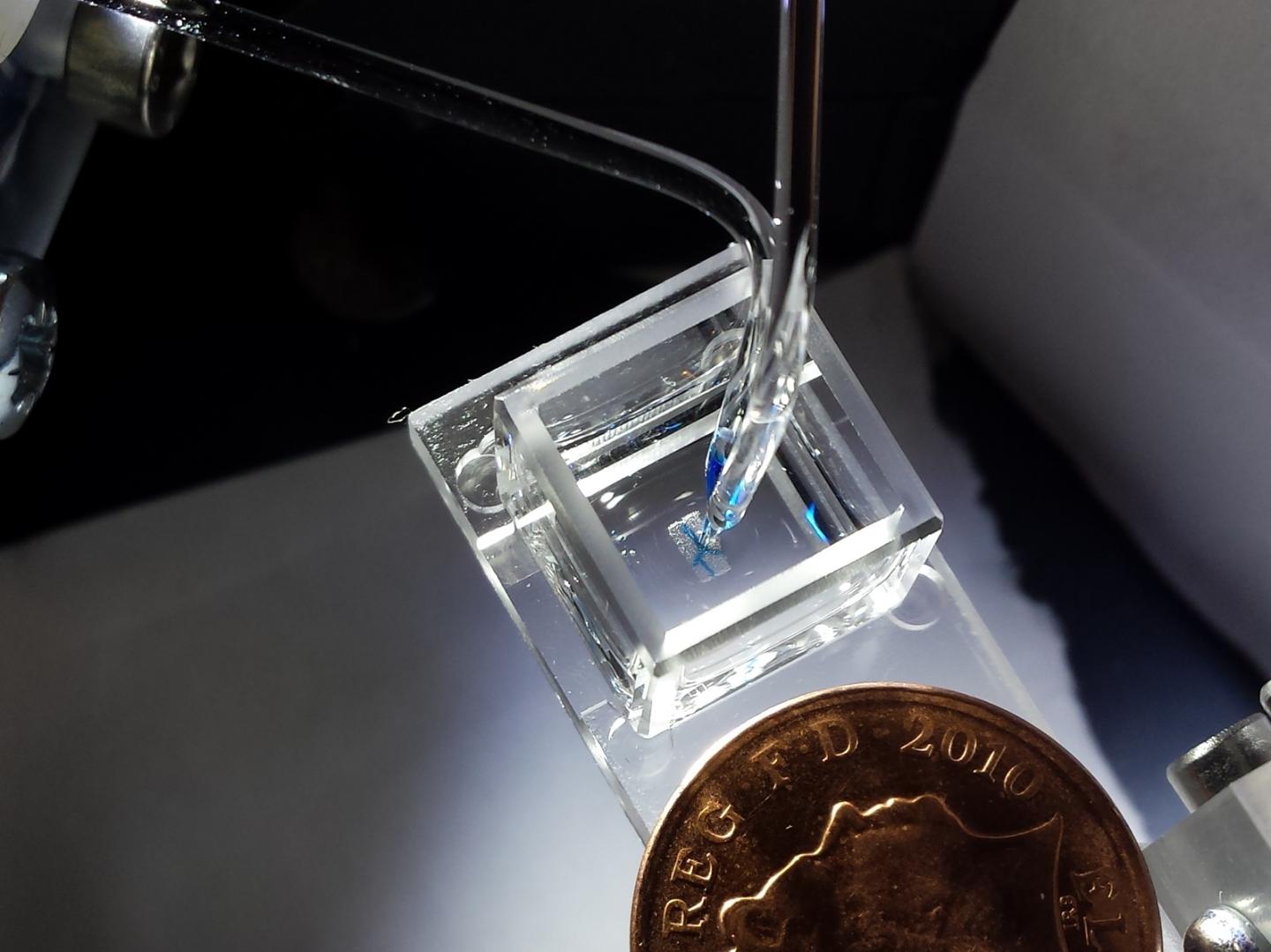
The 3D droplet bioprinter, developed by the Bayley Research Group at Oxford, producing millimeter-sized tissues (credit: Sam Olof/ Alexander Graham)
Scientists at the University of Oxford have developed a radical new method of 3D-printing laboratory-grown cells that can form complex living tissues and cartilage to potentially support, repair, or augment diseased and damaged areas of the body.
Printing high-resolution living tissues is currently difficult because the cells often move within printed structures and can collapse on themselves. So the team devised a new way to produce tissues in protective nanoliter droplets wrapped in a lipid (oil-compatible) coating that is assembled, layer-by-layer, into living cellular structures.
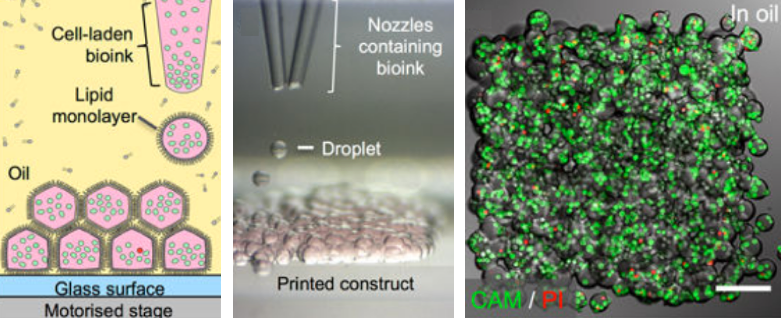
3D-printing cellular constructs. (left) Schematic of cell printing. The dispensing nozzle ejects cell-containing bioink droplets into a lipid-containing oil. The droplets are positioned by the programmed movement of the oil container. The droplets cohere through the formation of droplet interface lipid bilayers. (center) A related micrograph of a patterned cell junction, containing two cell types, printed as successive layers of 130-micrometer droplets ejected from two glass nozzles. (right) A confocal fluorescence micrograph of about 700 printed human embryonic kidney cells under oil at a density of 40 million cells per milliliter (scale bar = 150 micrometers). (credit: Alexander D. Graham et al./Scientific Reports)
This new method improves the survival rate of the individual cells and allows for building each tissue one drop at a time to mimic the behaviors and functions of the human body. The patterned cellular constructs, once fully grown, can mimic or potentially enhance natural tissues.
“We were aiming to fabricate three-dimensional living tissues that could display the basic behaviors and physiology found in natural organisms,” explained Alexander Graham, PhD, lead author and 3D Bioprinting Scientist at OxSyBio (Oxford Synthetic Biology).*
“To date, there are limited examples of printed tissues [that] have the complex cellular architecture of native tissues. Hence, we focused on designing a high-resolution cell printing platform, from relatively inexpensive components, that could be used to reproducibly produce artificial tissues with appropriate complexity from a range of cells, including stem cells.”
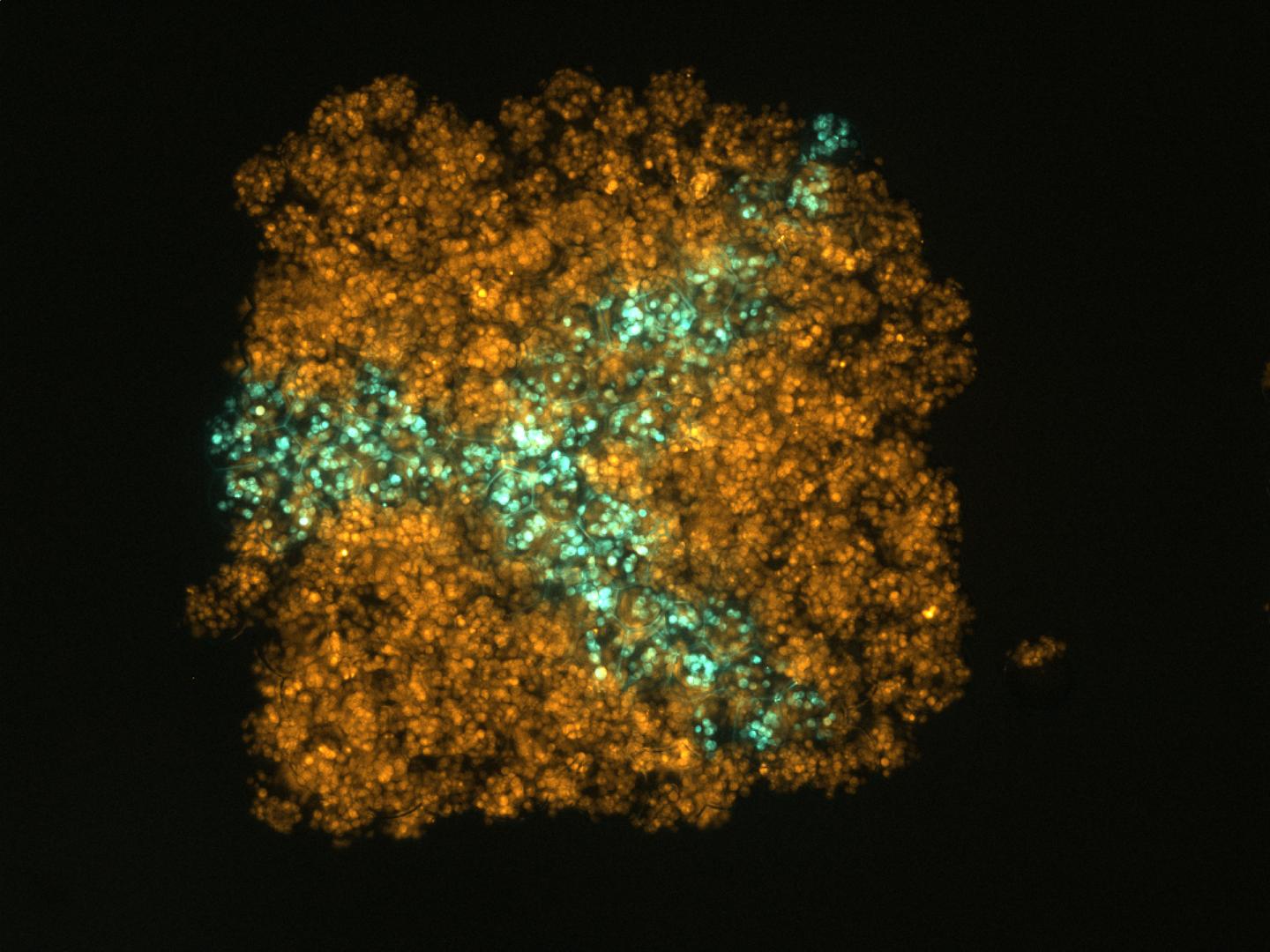
A confocal micrograph of an artificial tissue containing two populations of human embryonic kidney cells (HEK-293T) printed in the form of an arborized structure within a cube (credit: Sam Olof/Alexander Graham)
The researchers hope that with further development, the materials could have a wide impact on healthcare worldwide and bypass clinical animal testing. The scientists plan to develop new complementary printing techniques that allow for a wider range of living and hybrid materials, producing tissues at industrial scale.
“We believe it will be possible to create personalized treatments by using cells sourced from patients to mimic or enhance natural tissue function,” said Sam Olof, PhD, Chief Technology Officer at OxSyBio. “In the future, 3D bio-printed tissues may also be used for diagnostic applications — for example, for drug or toxin screening.”
The study results were published August 1 in the open-access journal Scientific Reports.
Abstract of High-Resolution Patterned Cellular Constructs by Droplet-Based 3D Printing
Bioprinting is an emerging technique for the fabrication of living tissues that allows cells to be arranged in predetermined three-dimensional (3D) architectures. However, to date, there are limited examples of bioprinted constructs containing multiple cell types patterned at high-resolution. Here we present a low-cost process that employs 3D printing of aqueous droplets containing mammalian cells to produce robust, patterned constructs in oil, which were reproducibly transferred to culture medium. Human embryonic kidney (HEK) cells and ovine mesenchymal stem cells (oMSCs) were printed at tissue-relevant densities (107 cells mL−1) and a high droplet resolution of 1 nL. High-resolution 3D geometries were printed with features of ≤200 μm; these included an arborised cell junction, a diagonal-plane junction and an osteochondral interface. The printed cells showed high viability (90% on average) and HEK cells within the printed structures were shown to proliferate under culture conditions. Significantly, a five-week tissue engineering study demonstrated that printed oMSCs could be differentiated down the chondrogenic lineage to generate cartilage-like structures containing type II collagen.