A fast, accurate, nanoscale ‘biochemical nose’ sensor
August 5, 2015
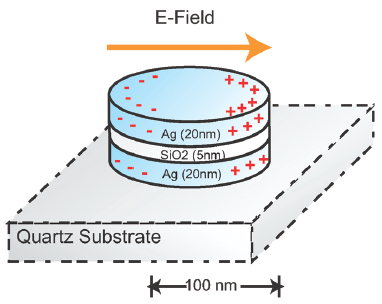
A nanoplasmonic resonator (NPR) consists of a thin silicon dioxide layer sandwiched between metallic nanodisks. NPRs can enhance surface-enhanced Raman spectroscopic (SERS) signals by a factor of 60 billion to detect target molecules with high sensitivity. (credit: Cheng Sun et al./ ACS Nano)
Imagine being able to test your food in your kitchen to quickly determine if it carried any deadly microbes. Technology now being commercialized by Optokey may soon make that possible.
Optokey, a startup based in Hayward, California, has developed a miniaturized sensor using surface-enhanced Raman spectroscopy (SERS) that can quickly and accurately detect or diagnose substances at a molecular level. The technology is based on research conducted at Lawrence Berkeley National Laboratory (Berkeley Lab) and published in 2010.
Molecular fingerprinting
“Our system can do chemistry, biology, biochemistry, molecular biology, clinical diagnosis, and chemical analysis,” said Optokey president and co-founder Fanqing Frank Chen, a scientist at Berkeley Lab who was co-author of an ACS Nano paper on the research. The system can be implemented “very cheaply, without much human intervention,” he said.
SERS is a highly sensitive analytical tool used for “molecular fingerprinting,” but the results have not easily reproducible. Chen and colleagues developed a solution to this problem using what they called “nanoplasmonic resonators,” which measures the interaction of photons with an activated surface using nanostructures to do chemical and biological sensing. The method produces measurements much more reliably.
“At Optokey we’re able to mass produce this nanoplasmonic resonator on a wafer scale,” Chen said. “We took something from the R&D realm and turned it into something industrial-strength.”
The miniaturized sensors use a microfluidic control system for “lab on a chip” automated liquid sampling. “We’re leveraging knowledge acquired from high-tech semiconductor manufacturing methods to get the cost, the volume, and the accuracy in the chip,” said VP of Manufacturing Robert Chebi, a veteran of the microelectronic industry who previously worked at Lam Research and Applied Materials. “We’re also leveraging all the knowledge in lasers and optics for this specific Raman-based method.”
A biochemical nose
Chebi calls Optokey’s product a “biochemical nose,” or an advanced nanophotonic automated system, with sensitivity to the level of a single molecule, far superior to sensors on the market today, he claims. “Today’s detection and diagnosis methods are far from perfect … Also, our system can provide information in minutes, or even on a continuous basis, versus other methods where it could take hours or even days, if samples have to be sent to another lab.”
The potential applications include food safety, environmental monitoring (of both liquids and gases), medical diagnosis, and chemical analysis. Optokey’s customers include a major European company interested in food safety, a Chinese petrochemical company interested in detecting impurities in its products, and a German company interested in point-of-care diagnosis.
“The product we’re envisioning is something that is compact and automated but also connected, and it can go into schools, restaurants, factories, hospitals, ambulances, airports, and even battlefields,” Chen said. Next, they plan to introduce it in the smart home, where a nanophotonic sensor could be built to scan for pollutants not just in food but also in air and water.
Key discovery: nanoplasmonic resonators
Ultimately, Chen and his Berkeley Lab group developed about 20 patents involving hybrid bionanomaterials. The key discovery that led to the formation of Optokey was the development of nanoplasmonic resonators to dramatically improve the signal and reliability of Raman spectroscopy. The method was initially used in the research lab to quickly and accurately detect a biomarker for prostate cancer, which has a high rate of false positives using conventional diagnostic tools.
“There was 10 years of research that went into this, funded by NIH, DARPA, the federal government, private foundations,” said Chen. “Berkeley Lab has a really good culture of multidisciplinary research, excellent engineering, and very strong basic science. Plus it has strong support for startups.”
Abstract of Time-Resolved Single-Step Protease Activity Quantification Using Nanoplasmonic Resonator Sensors
Protease activity measurement has broad application in drug screening, diagnosis and disease staging, and molecular profiling. However, conventional immunopeptidemetric assays (IMPA) exhibit low fluorescence signal-to-noise ratios, preventing reliable measurements at lower concentrations in the clinically important picomolar to nanomolar range. Here, we demonstrated a highly sensitive measurement of protease activity using a nanoplasmonic resonator (NPR). NPRs enhance Raman signals by 6.1 × 1010 times in a highly reproducible manner, enabling fast detection of proteolytically active prostate-specific antigen (paPSA) activities in real-time, at a sensitivity level of 6 pM (0.2 ng/mL) with a dynamic range of 3 orders of magnitude. Experiments on extracellular fluid (ECF) from the paPSA-positive cells demonstrate specific detection in a complex biofluid background. This method offers a fast, sensitive, accurate, and one-step approach to detect the proteases’ activities in very small sample volumes.