Artificial cells mimic natural protein synthesis
August 19, 2014
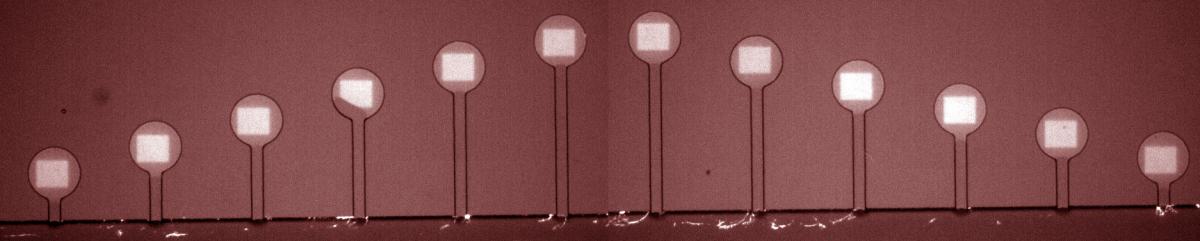
Fluorescent image of DNA (white squares) patterned in circular compartments connected by capillary tubes to the cell-free extract flowing in the channel at bottom. Compartments are 100 microns in diameter. (Credit: Weizmann Institute)
Weizmann Institute scientists have created an artificial network-like cell system that is capable of reproducing the dynamic behavior of protein synthesis.
This achievement could help gain a deeper understanding of basic biological processes and pave the way toward controlling the synthesis of naturally occurring and synthetic proteins for many uses.
The system was designed by PhD students Eyal Karzbrun and Alexandra Tayar in the lab of Prof. Roy Bar-Ziv of the Weizmann Institute’s Materials and Interfaces Department, in collaboration with Prof. Vincent Noireaux of the University of Minnesota,
How it works
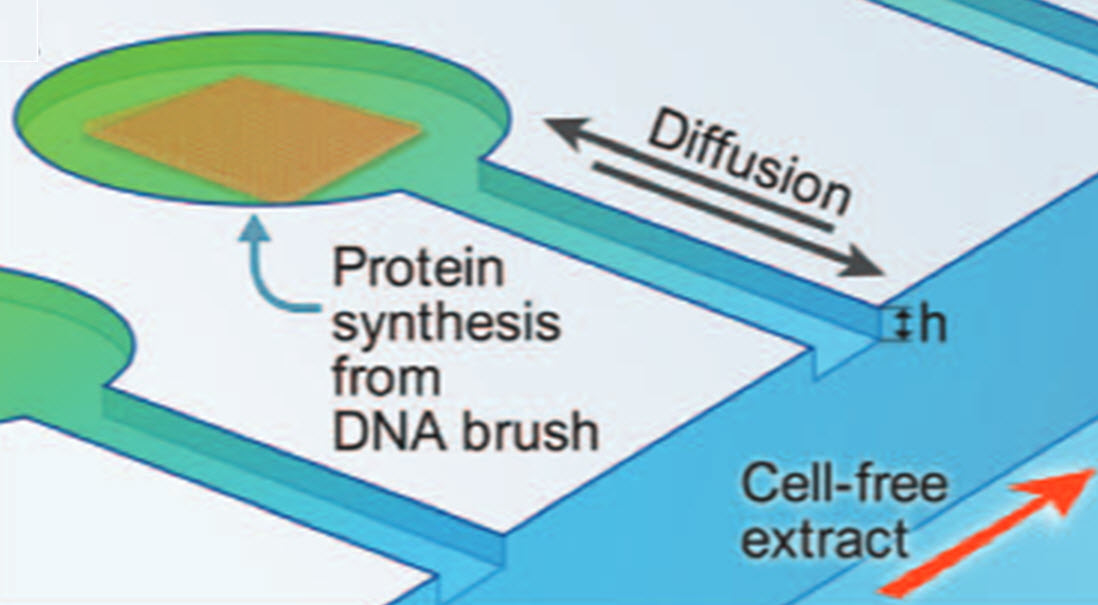
DNA template (DNA brush) is assembled by chemical photolithography, patterned in circular compartments carved in silicon, and connected through a diffusive capillary to a channel flowing a cell-free expression reaction (credit: Eyal Karzbrun et al./Science)
1. Multiple artificial cells (compartments), each a micron (millionth of a meter) in depth, are “etched’’ onto a biochip.
2. The compartments are connected via thin capillary tubes, creating a network that allows for diffusion of biological substances throughout the system.
3. Within each compartment, the researchers insert a cell genome — strands of DNA designed and controlled by the scientists.
4. To translate (convert) the genes into proteins, the scientists relinquish control to the bacterium E. coli: they fill the compartments with E. coli cell extract — a solution containing the entire bacterial protein-translating machinery, minus its DNA code.
By coding two regulatory genes into the sequence (step 3), the scientists create a protein synthesis rate that is periodic, spontaneously switching from periods of being “on” to “off.” The amount of time each period lasts is determined by the geometry of the compartments.
Such periodic behavior — a primitive version of cell cycle events — emerges in the system because the synthesized proteins can diffuse out of the compartment through the capillaries, mimicking natural protein turnover behavior in living cells. At the same time, fresh nutrients are continuously replenished, diffusing into the compartment and enabling the protein synthesis reaction to continue indefinitely.
“The artificial cell system, in which we can control the genetic content and protein dilution times, allows us to study the relation between gene network design and the emerging protein dynamics. This is quite difficult to do in a living system,” says Karzbrun.
More complicated gene networks
“The two-gene pattern we designed is a simple example of a cell network, but after proving the concept, we can now move forward to more complicated gene networks. One goal is to eventually design DNA content similar to a real genome that can be placed in the compartments.”
The scientists then asked whether the artificial cells actually communicate and interact with one another like real cells. Indeed, they found that the synthesized proteins that diffused through the array of interconnected compartments were able to regulate genes and produce new proteins in compartments farther along the network.
In fact, this system resembles the initial stages of morphogenesis — the biological process that governs the emergence of the body plan in embryonic development. “We observed that when we place a gene in a compartment at the edge of the array, it creates a diminishing protein concentration gradient; other compartments within the array can sense and respond to this gradient — similar to how morphogen concentration gradients diffuse through the cells and tissues of an embryo during early development.
We are now working to expand the system and to introduce gene networks that will mimic pattern formation, such as the striped patterns that appear during fly embryogenesis,” explains Tayar.
With the artificial cell system, according to Bar-Ziv, one can, in principle, encode anything: “Genes are like Lego in which you can mix and match various components to produce different outcomes; you can take a regulatory element from E. coli that naturally controls gene X, and produce a known protein; or you can take the same regulatory element but connect it to gene Y instead to get different functions that do not naturally occur in nature.”
This research may, in the future, help advance the synthesis of such things as fuel, pharmaceuticals, chemicals and the production of enzymes for industrial use.
Prof. Roy Bar-Ziv’s research is supported by the Yeda-Sela Center for Basic Research.
Abstract of Science paper
The assembly of artificial cells capable of executing synthetic DNA programs has been an important goal for basic research and biotechnology. We assembled two-dimensional DNA compartments fabricated in silicon as artificial cells capable of metabolism, programmable protein synthesis, and communication. Metabolism is maintained by continuous diffusion of nutrients and products through a thin capillary, connecting protein synthesis in the DNA compartment with the environment. We programmed protein expression cycles, autoregulated protein levels, and a signaling expression gradient, equivalent to a morphogen, in an array of interconnected compartments at the scale of an embryo. Gene expression in the DNA compartment reveals a rich, dynamic system that is controlled by geometry, offering a means for studying biological networks outside a living cell.