Buckyballs and diamondoids combine to form basic electronic device
September 12, 2014
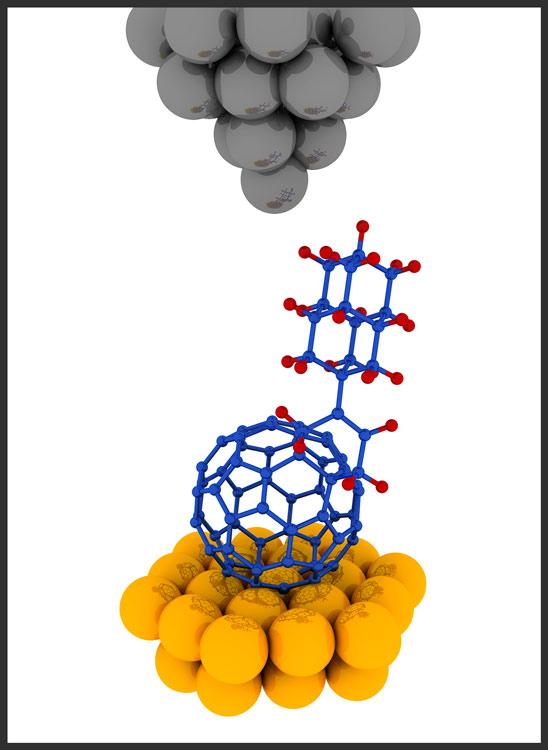
Illustration of a “buckydiamondoid” molecule under a scanning tunneling microscope (gray and yellow spheres) (credit: Stanford University)
This tiny electronic component could play a key role in shrinking chip components down to the size of molecules to enable faster, more powerful devices.
“We wanted to see what new, emergent properties might come out when you put these two ingredients together to create a ‘buckydiamondoid,'” said Hari Manoharan of the Stanford Institute for Materials and Energy Sciences (SIMES) at the U.S. Department of Energy’s SLAC National Accelerator Laboratory.
“What we got was basically a one-way valve for conducting electricity.”
The research team, which included scientists from Stanford University, Belgium, Germany and Ukraine, reported its results Sept. 9 in Nature Communications (open access).
Many electronic circuits have three basic components: a material that conducts electrons; rectifiers, which commonly take the form of diodes, to steer that flow in a single direction; and transistors to switch the flow on and off.
Buckyballs — short for buckminsterfullerenes — are hollow carbon spheres whose 1985 discovery earned three scientists a Nobel Prize in chemistry. Diamondoids are tiny linked cages of carbon joined, or bonded, as they are in diamonds, with hydrogen atoms linked to the surface, but weighing less than a billionth of a billionth of a carat. Both are subjects of a lot of research aimed at understanding their properties and finding ways to use them.
In 2007, a team led by researchers from SLAC and Stanford discovered that a single layer of diamondoids on a metal surface can emit and focus electrons into a tiny beam. Manoharan and his colleagues wondered: What would happen if they paired an electron-emitting diamondoid with another molecule that likes to grab electrons?
A valve for channeling electron flow
They discovered that the hybrid is an excellent rectifier: the electrical current flowing through the molecule was up to 50 times stronger in one direction, from electron-spitting diamondoid to electron-catching buckyball, than in the opposite direction.
While this is not the first molecular rectifier ever invented, it’s the first one made from just carbon and hydrogen, a simplicity researchers find appealing, said Manoharan, who is an associate professor of physics at Stanford. The next step, he said, is to see if transistors can be constructed from the same basic ingredients.
“Buckyballs are easy to make — they can be isolated from soot — and the type of diamondoid we used here, which consists of two tiny cages, can be purchased commercially,” he said. “And now that our colleagues in Germany have figured out how to bind them together, others can follow the recipe. So while our research was aimed at gaining fundamental insights about a novel hybrid molecule, it could lead to advances that help make molecular electronics a reality.”
Other research collaborators came from the Catholic University of Louvain in Belgium and Kiev Polytechnic Institute in Ukraine. The primary funding for the work came from U.S. the Department of Energy Office of Science (Basic Energy Sciences, Materials Sciences and Engineering Divisions).
Abstract of Nature Communications paper
The unimolecular rectifier is a fundamental building block of molecular electronics. Rectification in single molecules can arise from electron transfer between molecular orbitals displaying asymmetric spatial charge distributions, akin to p–n junction diodes in semiconductors. Here we report a novel all-hydrocarbon molecular rectifier consisting of a diamantane–C60 conjugate. By linking both sp3 (diamondoid) and sp2 (fullerene) carbon allotropes, this hybrid molecule opposingly pairs negative and positive electron affinities. The single-molecule conductances of self-assembled domains on Au(111), probed by low-temperature scanning tunnelling microscopy and spectroscopy, reveal a large rectifying response of the molecular constructs. This specific electronic behaviour is postulated to originate from the electrostatic repulsion of diamantane–C60 molecules due to positively charged terminal hydrogen atoms on the diamondoid interacting with the top electrode (scanning tip) at various bias voltages. Density functional theory computations scrutinize the electronic and vibrational spectroscopic fingerprints of this unique molecular structure and corroborate the unconventional rectification mechanism.