Building customized DNA nanotubes step by step
February 24, 2015
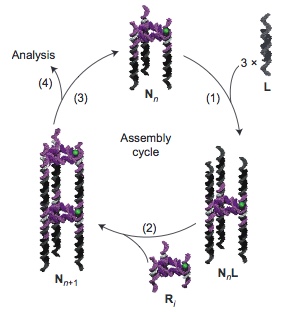
Schematic representation of the stepwise assembly of a triangular-shaped DNA nanotube (N). The time-programmed cyclic sequence involves the sequential addition first of prefabricated DNA linkers (L) (step 1) and then of triangular rungs (R) (step2) — both consisting of duplex DNA — to the nanotube by flowing, incubating and washing. This cycle goes on (step 3) to produce the desired nanotube, which is then characterized by single-molecule spectroscopy of fluorescently tagged (green in this example) rungs. (credit: A. Hariri et al./Nature Chemistry)
McGill University researchers have developed a new low-cost method to build DNA nanotubes block by block. It could help pave the way for scaffolds made from DNA strands for applications such as optical and electronic devices or smart drug-delivery systems.
The current method of constructing DNA nanotubes is based on spontaneous assembly of DNA in solution, which is vulnerable to structural flaws.
The new technique, reported Monday Feb. 23 in Nature Chemistry, promises to reduce such flaws and also makes it possible to better control the size and patterns of the DNA structures, the scientists report.
“Just like a Tetris game, where we manipulate the game pieces with the aim of creating a horizontal line of several blocks, we can now build long nanotubes block by block,” said Amani Hariri, a PhD student in McGill’s Department of Chemistry and lead author of the study.
“By using a fluorescence microscope we can further visualize the formation of the tubes at each stage of assembly, as each block is tagged with a fluorescent compound that serves as a beacon. We can then count the number of blocks incorporated in each tube as it is constructed.”
This new technique was made possible by the development in recent years of single-molecule microscopy.
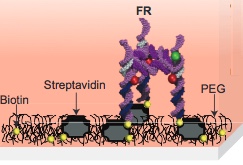
Molecular self-assembly of a foundation rung (FR) labeled with two fluorophores (red and green) (credit: A. Hariri et al./Nature Chemistry)
That research has enabled scientists to view at the nanoscale by turning the fluorescence of individual molecules on and off. (That groundbreaking work won three U.S.- and German-based scientists the 2014 Nobel Prize in Chemistry.)
The resulting “designer nanotubes” approach promises to be far cheaper to produce on a large scale than those created with DNA origami — another technique for using DNA as a nanoscale construction material — according to Hanadi Sleiman, who co-authored the new study and holds the Canada Research Chair in DNA Nanoscience.
Funding for the research was provided by the Natural Sciences and Engineering Research Council of Canada, the Canada Foundation for Innovation, NanoQuébec, the Canadian Institutes of Health Research, and the Fonds de recherché du Québec – Nature et technologies.
Abstract of Stepwise growth of surface-grafted DNA nanotubes visualized at the single-molecule level
DNA nanotubes offer a high aspect ratio and rigidity, attractive attributes for the controlled assembly of hierarchically complex linear arrays. It is highly desirable to control the positioning of rungs along the backbone of the nanotubes, minimize the polydispersity in their manufacture and reduce the building costs. We report here a solid-phase synthesis methodology in which, through a cyclic scheme starting from a ‘foundation rung’ specifically bound to the surface, distinct rungs can be incorporated in a predetermined manner. Each rung is orthogonally addressable. Using fluorescently tagged rungs, single-molecule fluorescence studies demonstrated the robustness and structural fidelity of the constructs and confirmed the incorporation of the rungs in quantitative yield (>95%) at each step of the cycle. Prototype structures that consisted of up to 20 repeat units, about 450 nm in contour length, were constructed. Combined, the solid-phase synthesis strategy described and its visualization through single-molecule spectroscopy show good promise for the production of custom-made DNA nanotubes.