Cancer cells in 3D
February 22, 2016

A spheroid of many lung cancer cells illustrates a diversity of behaviors. (credit: Welf and Driscoll et al./Developmental Cell)
Cancer cells don’t live on glass slides. Yet the vast majority of images related to cancer biology come from the cells being photographed on flat, two-dimensional surfaces — images sometimes used to draw conclusions about the behavior of cells that normally reside in a more complex environment.
Now a new high-resolution microscope, presented (open access) February 22 in Developmental Cell, makes it possible to visualize cancer cells in 3D and record how they are signaling to other parts of their environment — revealing previously unappreciated biology of how cancer cells survive and disperse within living things. Based on “microenvironmental selective plane illumination microscopy” (meSPIM), the new microscope is designed to image cells in microenvironments free of hard surfaces near the sample.
“There is clear evidence that the environment strongly affects cellular behavior — thus, the value of cell culture experiments on glass must at least be questioned,” says senior author Reto Fiolka, an optical scientist at the University of Texas Southwestern Medical Center. “Our microscope is one tool that may bring us a deeper understanding of the molecular mechanisms that drive cancer cell behavior, since it enables high-resolution imaging in more realistic tumor environments.”
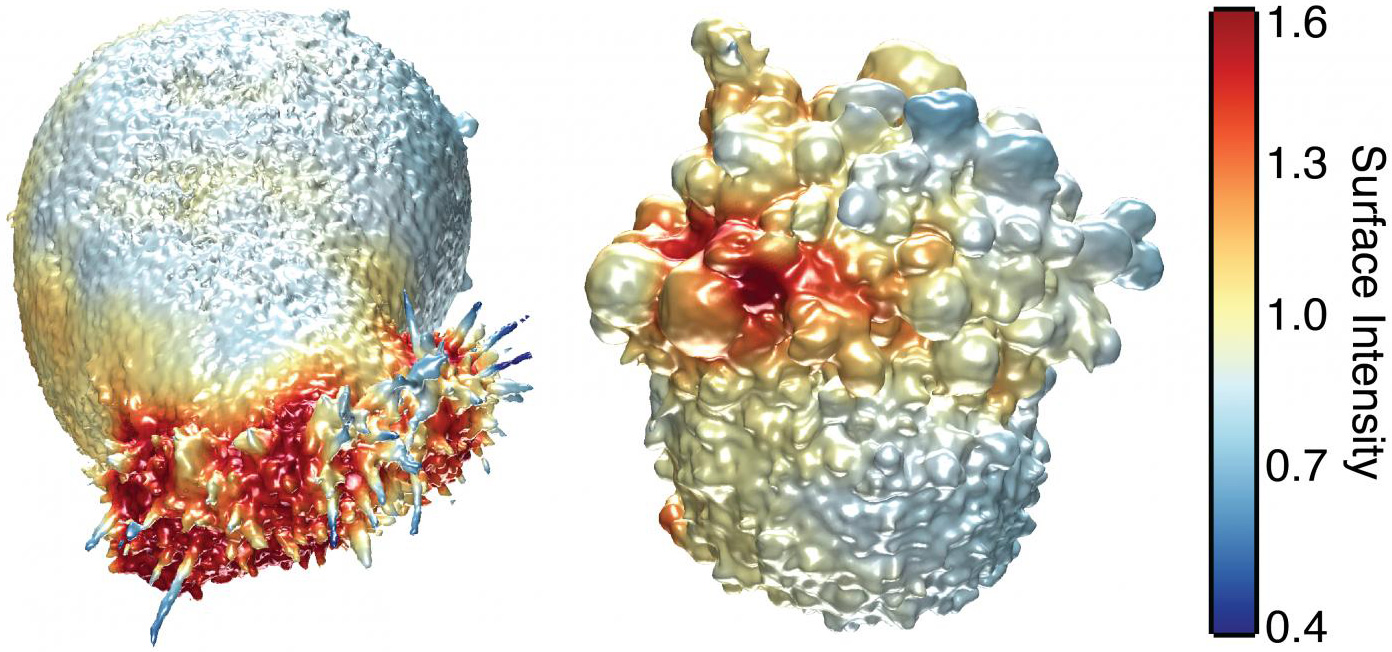
This image shows the extracted surfaces of two cancer cells. (Left) A lung cancer cell colored by actin intensity near the cell surface. Actin is a structural molecule that is integral to cell movement. (Right) A melanoma cell colored by PI3-kinase activity near the cell surface. PI3K is a signaling molecule that is key to many cell processes. (credit: Welf and Driscoll et al./Developmental Cell)
Hidden protrusions from cancer cells
In their study, Fiolka and colleagues, including co-senior author Gaudenz Danuser, and co-first authors Meghan Driscoll and Erik Welf, also of UT Southwestern, used their microscope to image different kinds of skin cancer cells from patients. They found that in a 3D environment (where cells normally reside), unlike a glass slide, multiple melanoma cell lines and primary melanoma cells (from patients with varied genetic mutations) form many small protrusions called blebs.
One hypothesis is that this blebbing may help the cancer cells survive or move around and could thus play a role in skin cancer cell invasiveness or drug resistance in patients.
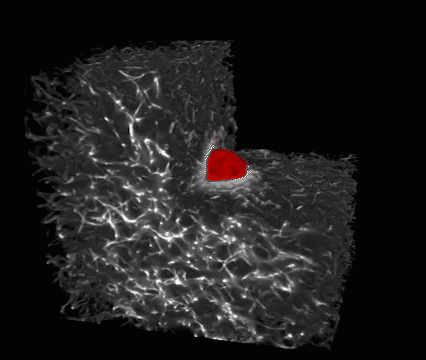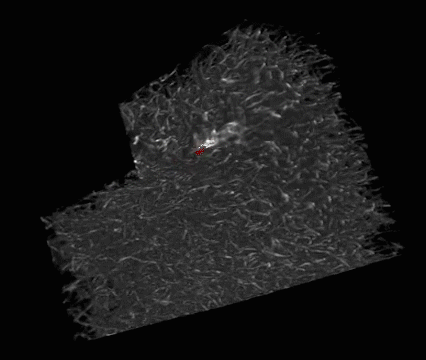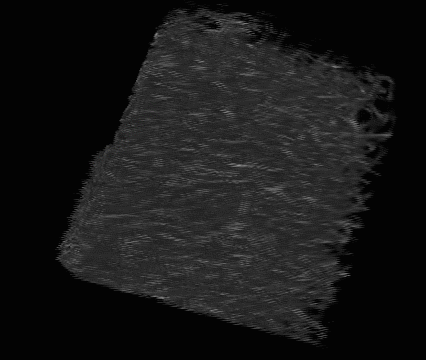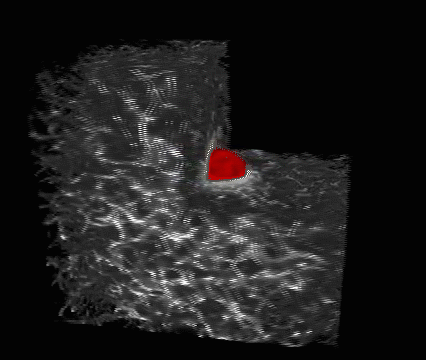
This is a melanoma cell (red) embedded in a 3-D collagen matrix (white). A 100 x 100 x 100 μm cube is shown, with one corner cut away to show the interaction of the cell with the collagen. (credit: Welf and Driscoll et al./Developmental Cell)
The researchers say that this is a first step toward understanding 3D biology in tumor microenvironments. But since these kinds of images may be too complicated to interpret by the naked eye alone, the next step will be to develop powerful computer platforms to extract and process the information.
The microscope control software and image analytical code are freely available to the scientific community.
The authors were supported by the Cancer Prevention Research Institute of Texas and the National Institutes of Health.
Abstract of Quantitative Multiscale Cell Imaging in Controlled 3D Microenvironments
The microenvironment determines cell behavior, but the underlying molecular mechanisms are poorly understood because quantitative studies of cell signaling and behavior have been challenging due to insufficient spatial and/or temporal resolution and limitations on microenvironmental control. Here we introduce microenvironmental selective plane illumination microscopy (meSPIM) for imaging and quantification of intracellular signaling and submicrometer cellular structures as well as large-scale cell morphological and environmental features. We demonstrate the utility of this approach by showing that the mechanical properties of the microenvironment regulate the transition of melanoma cells from actin-driven protrusion to blebbing, and we present tools to quantify how cells manipulate individual collagen fibers. We leverage the nearly isotropic resolution of meSPIM to quantify the local concentration of actin and phosphatidylinositol 3-kinase signaling on the surfaces of cells deep within 3D collagen matrices and track the many small membrane protrusions that appear in these more physiologically relevant environments.