Complex 3D physical tissue model simulates live cortex biochemical and electrical behavior
August 15, 2014

Silk 3D brain tissue model of the six neocortex layers, dyed with food color (credit: Tufts University)
Tufts University researchers have developed the first reported complex three-dimensional model made of material that simulates cortical tissue’s biochemical and electrophysiological responses.
Funded by the National Institutes of Health, the engineered tissue model offers new options for studying brain function, disease and trauma, and treatment, and can function in the laboratory for months.
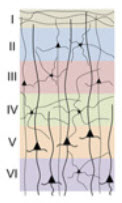
Neocortex layers (credit: Min D. Tang-Schomer et al./PNAS)
Creating a 3D model of neocortex tissue
Circular modules of cast silk were punched into “doughnuts,” then assembled into concentric rings to simulate the six layers of the neocortex. Each layer was seeded with neurons independently before assembly, without the need for adhesive or glue.
The doughnuts were then immersed in a softer collagen gel matrix that allowed the neurons’ axons to penetrate and connect three-dimensionally.
The researchers said this silk-collagen gel combination provided an optimum microenvironment for neural-network formation and function.
“The stiffness of the silk biomaterial could be tuned to accommodate the cortical neurons and the different types of gels, maintaining both stability in culture and brain-like tissue elasticity,” said the paper’s first author, Min D. Tang-Schomer, Ph.D., post-doctoral scholar in biomedical engineering at Tufts.
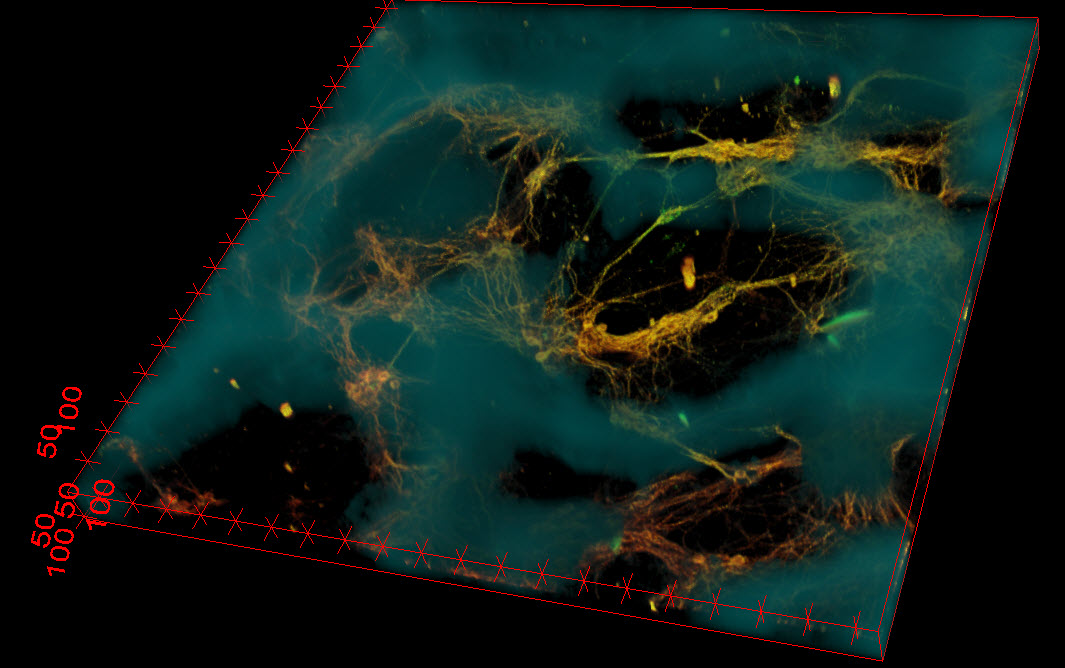
Confocal microscope image of neurons (greenish yellow) attached to a small portion of the silk-based scaffold (blue). The neurons formed functional networks throughout the scaffold pores (dark areas). (Credit: Tufts University)
“The tissue maintained viability for at least nine weeks — significantly longer than cultures made of collagen or hydrogel alone — and also offered structural support for network connectivity that is crucial for brain activity.”
Studying Brain Damage
The Tufts researchers were able to use the tissue model to examine multiple post-injury effects, including cellular damage, electrophysiological activity, and neurochemical changes.
For example, when a weight was dropped on the model tissue to simulate a traumatic brain injury, the tissue released high levels of the chemical glutamate, a neurotransmitter known to be emitted by cells following brain damage.
The tissue also showed transient electrical hyperactivity consistent with post-trauma responses observed in vivo (in live brains).
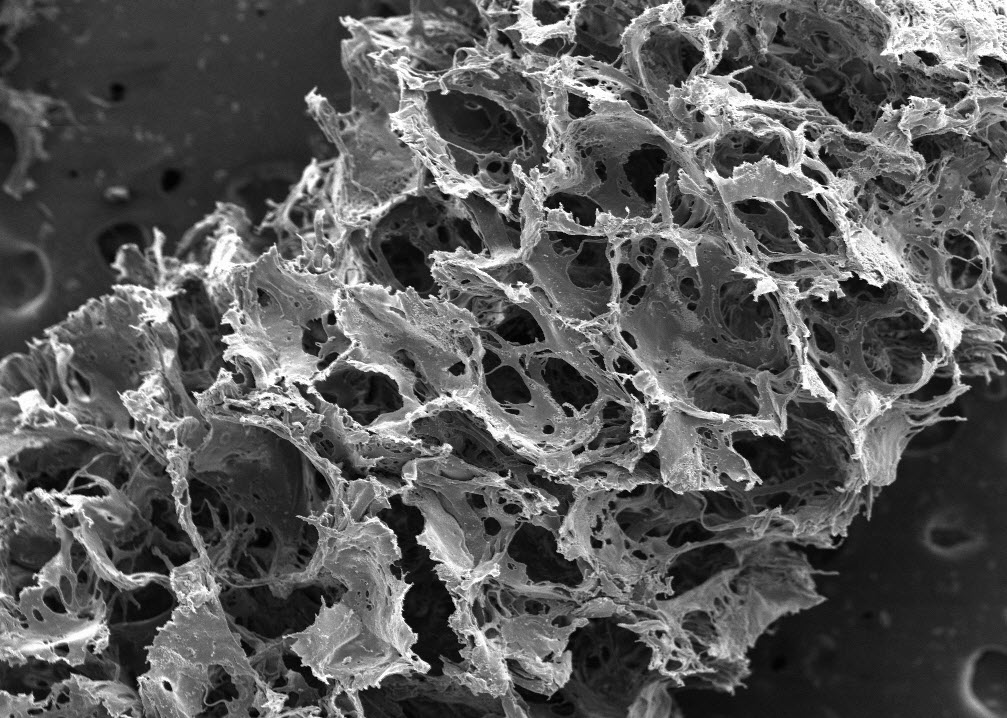
Image of silk-based scaffold taken with a scanning electron microscope reveals its porous, sponge-like composition. (Credit: Tufts University)
“This model provides a unique opportunity for mapping out real-time neurophysiological events and function studies in the laboratory, monitoring that is prohibitive with humans or animals,” said study co-author Philip Haydon, Ph.D., Annetta and Gustav Grisard professor of neuroscience at the Sackler School of Graduate Biomedical Sciences, Tufts University School of Medicine.
Work is underway to further develop the model, said co-author David Kaplan, Ph.D., who directs the NIH-funded P41 Tissue Engineering Resource Center based at Tufts. It could potentially be applied to study brain structure and function, drug screening, impact of electrodes and implants on brain function, disease formation and treatments, and the effects of nutrition and toxicants.
Abstract of Proceedings of the National Academy of Sciences paper
The brain remains one of the most important but least understood tissues in our body, in part because of its complexity as well as the limitations associated with in vivo studies. Although simpler tissues have yielded to the emerging tools for in vitro 3D tissue cultures, functional brain-like tissues have not. We report the construction of complex functional 3D brain-like cortical tissue, maintained for months in vitro, formed from primary cortical neurons in modular 3D compartmentalized architectures with electrophysiological function. We show that, on injury, this brain-like tissue responds in vitro with biochemical and electrophysiological outcomes that mimic observations in vivo. This modular 3D brain-like tissue is capable of real-time nondestructive assessments, offering previously unidentified directions for studies of brain homeostasis and injury.