Controlled by a synthetic gene circuit, self-assembling bacteria build working electronic sensors
October 11, 2017
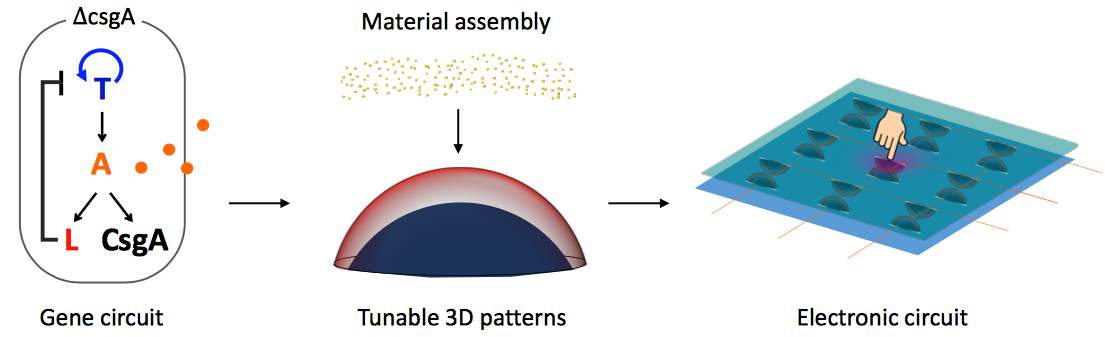
Bacteria create a functioning 3D pressure-sensor device. A gene circuit (left) triggers the production of an engineered protein that enables pattern-forming bacteria on growth membranes (center) to assemble gold nanoparticles into a hybrid organic-inorganic dome structure whose size and shape can be controlled by altering the growth environment. In this proof-of-concept demonstration, the gold structure serves as a functioning pressure switch (right) that responds to touch. (credit: Yangxiaolu Cao et al./Nature Biotechnology)
Using a synthetic gene circuit, Duke University researchers have programmed self-assembling bacteria to build useful electronic devices — a first.
Other experiments have successfully grown materials using bacterial processes (for example, MIT engineers have coaxed bacterial cells to produce biofilms that can incorporate nonliving materials, such as gold nanoparticles and quantum dots). However, they have relied entirely on external control over where the bacteria grow and they have been limited to two dimensions.
In the new study, the researchers demonstrated the production of a composite structure by programming the cells themselves and controlling their access to nutrients, but still leaving the bacteria free to grow in three dimensions.*
As a demonstration, the bacteria were programmed to assemble into a finger-pressure sensor.
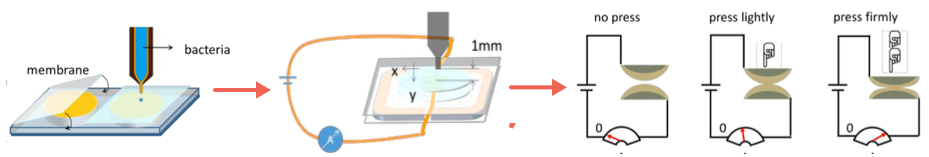
To create the pressure sensor, two identical arrays of domes were grown on a membrane (left) on two substrate surfaces. The two substrates were then sandwiched together (center) so that each dome was positioned directly above its counterpart on the other substrate. A battery was connected to the domes by copper wiring. When pressure was applied (right) to the sandwich, the domes pressed into one another, causing a deformation, resulting in an increase in conductivity, with resulting increased current (as shown the arrow in the ammeter). (credit: Yangxiaolu Cao et al./Nature Biotechnology)
Inspired by nature, but going beyond it
“This technology allows us to grow a functional device from a single cell,” said Lingchong You, the Paul Ruffin Scarborough Associate Professor of Engineering at Duke. “Fundamentally, it is no different from programming a cell to grow an entire tree.”
Nature is full of examples of life combining organic and inorganic compounds to make better materials. Mollusks grow shells consisting of calcium carbonate interlaced with a small amount of organic components, resulting in a microstructure three times tougher than calcium carbonate alone. Our own bones are a mix of organic collagen and inorganic minerals made up of various salts.
Harnessing such construction abilities in bacteria would have many advantages over current manufacturing processes. In nature, biological fabrication uses raw materials and energy very efficiently. In this synthetic system, for example, tweaking growth instructions to create different shapes and patterns could theoretically be much cheaper and faster than casting the new dies or molds needed for traditional manufacturing.
“Nature is a master of fabricating structured materials consisting of living and non-living components,” said You. “But it is extraordinarily difficult to program nature to create self-organized patterns. This work, however, is a proof-of-principle that it is not impossible.”
Self-healing materials
According to the researchers, in addition to creating circuits from bacteria, if the bacteria are kept alive, it may be possible to create materials that could heal themselves and respond to environmental changes.
“Another aspect we’re interested in pursuing is how to generate much more complex patterns,” said You. “Bacteria can create complex branching patterns, we just don’t know how to make them do that ourselves — yet.”
It’s a “very exciting work,” Timothy Lu, a synthetic biologist at MIT, who was not involved in the research, told The Register. “I think this represents a major step forward in the field of living materials.” Lu believes self-assembling materials “could create new manufacturing processes that may use less energy or be better for the environment than the ones today,” the article said. “But ‘the design rules for enabling bottoms-up assembly of novel materials are still not well understood,’ he cautioned.”
The study appeared online on October 9, 2107 in Nature Biotechnology. This study was supported by the Office of Naval Research, the National Science Foundation, the Army Research Office, the National Institutes of Health, the Swiss National Science Foundation, and a David and Lucile Packard Fellowship.
* The gene circuit is like a biological package of instructions that researchers embed into a bacterium’s DNA. The directions first tell the bacteria to produce a protein called T7 RNA polymerase (T7RNAP), which then activates its own expression in a positive feedback loop. It also produces a small molecule called AHL that can diffuse into the environment like a messenger. As the cells multiply and grow outward, the concentration of the small messenger molecule hits a critical concentration threshold, triggering the production of two more proteins called T7 lysozyme and curli. The former inhibits the production of T7RNAP while the latter acts as sort of biological Velcro, which grabs onto gold nanoparticles supplied by the researchers, forming a dome shell (the structure of the sensor). The researchers were able to alter the size and shape of the dome by controlling the properties of the porous membrane it grows on. For example, changing the size of the pores or how much the membrane repels water affects how many nutrients are passed to the cells, altering their growth pattern.
Abstract of Programmed assembly of pressure sensors using pattern-forming bacteria
Conventional methods for material fabrication often require harsh reaction conditions, have low energy efficiency, and can cause a negative impact on the environment and human health. In contrast, structured materials with well-defined physical and chemical properties emerge spontaneously in diverse biological systems. However, these natural processes are not readily programmable. By taking a synthetic-biology approach, we demonstrate here the programmable, three-dimensional (3D) material fabrication using pattern-forming bacteria growing on top of permeable membranes as the structural scaffold. We equip the bacteria with an engineered protein that enables the assembly of gold nanoparticles into a hybrid organic-inorganic dome structure. The resulting hybrid structure functions as a pressure sensor that responds to touch. We show that the response dynamics are determined by the geometry of the structure, which is programmable by the membrane properties and the extent of circuit activation. Taking advantage of this property, we demonstrate signal sensing and processing using one or multiple bacterially assembled structures. Our work provides the first demonstration of using engineered cells to generate functional hybrid materials with programmable architecture.