DARPA selects research teams for its ElectRx neuron-sensing/stimulation program
October 6, 2015
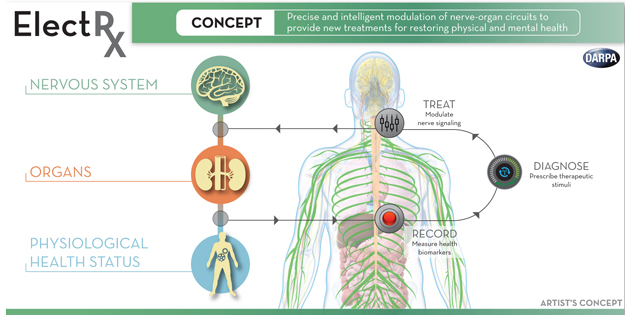
DARPA announced Monday (Oct. 5, 2015) that it has selected seven teams of researchers to begin work on a radical new approach to healing called Electrical Prescriptions (ElectRx). It would involve a system that stimulates peripheral nerves to modulate functions in the brain, spinal cord, and internal organs, according to program manager Doug Weber.
DARPA envisions a closed-loop system aimed at monitoring and treating conditions such as chronic pain, inflammatory disease, post-traumatic stress, and other illnesses that may not be responsive to traditional treatments, using optical, acoustic, electromagnetic, or engineered biology strategies to achieve precise targeting, possibly at single-axon resolution.
Pacemakers for other organs
The oldest and simplest example of this concept is the cardiac pacemaker, which uses brief pulses of electricity to stimulate the heart to beat at a healthy rate. DARPA aims to extend this concept to other organs, like the spleen, and treat inflammatory diseases such as rheumatoid arthritis.
Fighting inflammation may also provide new treatments for depression, which growing evidence suggests might be caused in part by excess levels of inflammatory biomolecules. Peripheral nerve stimulation may also be used to regulate production of neurochemicals that regulate learning and memory in the brain, offering new treatments for post-traumatic stress and other mental health disorders.
In phase 1, the ElectRx program will focus on fundamental studies to map the neural circuits governing the physiology of diseases of interest to DARPA, and also on preliminary development of novel, minimally invasive neural and bio-interface technologies with unprecedented levels of precision, targeting, and scale.
The teams
The seven teams include a mix of first-time and prior DARPA performers.
For example, an MIT team led by Polina Anikeeva will aim to advance its research in stimulating brain tissue using external magnetic fields and injected magnetic nanoparticles to treat neurological diseases such as Parkinson’s disease, replacing surgically implanted electrodes, as KurzweilAI reported in March. When exposed to a low-frequency (100 kHz — 1 MHz) external alternating magnetic field — which can penetrate deep inside biological tissues — these nanoparticles rapidly heat up and trigger heat-sensitive capsaicin (the “hot” in peppers) receptors to stimulate neurons.
MIT | Wireless brain stimulation
The other teams are:
- Circuit Therapeutics (Menlo Park, Calif.), a start-up co-founded by Stanford University scientists Karl Deisseroth and Scott Delp, plans to further develop its experimental optogenetic methods for treating neuropathic pain, building toward testing in animal models first.
- A team at Columbia University (New York), led by Elisa Konofagou, will pursue fundamental science to support the use of non-invasive, targeted ultrasound for neuromodulation. The team aims to elucidate the underlying mechanisms that may make ultrasound an option for chronic intervention, including activation and inhibition of nerves.
- A team at the Florey Institute of Neuroscience and Mental Health (Parkville, Australia), led by John Furness, will seek to map the nerve pathways that underlie intestinal inflammation, with a focus on determining the correlations between animal models and human neural circuitry. They will also explore the use of neurostimulation technologies based on the cochlear implant — developed by Cochlear, Inc. to treat hearing loss but adapted to modulate activity of the vagus nerve in response to biofeedback signals — as a possible treatment for inflammatory bowel disease.
- A team at the Johns Hopkins University (Baltimore), led by Jiande Chen, aims to explore the root mechanisms of inflammatory bowel disease and the impact of sacral nerve stimulation on its progression. The team will apply a first-of-its-kind approach to visualize intestinal responses to neuromodulation in animal models.
- A team at Purdue University (West Lafayette, Ind.), led by Pedro Irazoqui, will leverage an existing collaboration with Cyberonics to study inflammation of the gastrointestinal tract and its responsiveness to vagal nerve stimulation through the neck. Validation of the mechanistic insights that emerge from the effort will take place in pre-clinical models in which novel neuromodulation devices will be applied to reduce inflammation in a feedback-controlled manner. Later stages of the effort could advance the design of clinical neuromodulation devices.
- A team at the University of Texas, Dallas, led by Robert Rennaker and Michael Kilgard, will examine the use of vagal nerve stimulation to induce neural plasticity for the treatment of post-traumatic stress. As envisioned, stimulation could enhance learned behavioral responses that reduce fear and anxiety when presented with traumatic cues. Dr. Rennaker is a U.S. Marine Corps veteran who served in Liberia, Kuwait and Yugoslavia.