Electric current and antiobiotic kill multidrug-resistant bacteria in biofilms
November 30, 2016
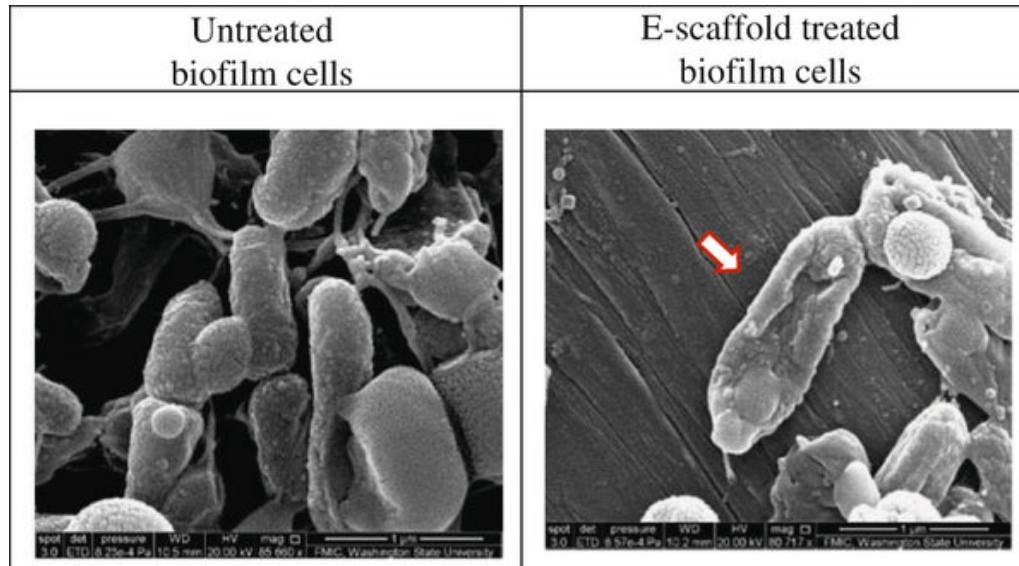
Washington State University researchers have successfully used a mild electric current to kill drug-resistant bacterial infections, a technology that may eventually be used to treat chronic wound infections. Red arrow indicates cell showing a stressed membrane. (credit: Washington State University)
A Washington State University research team has successfully used a mild electric current combined with an antibiotic to kill multidrug-resistant pseudomonas aeruginosa PAO1 bacteria in a lab-cultured biofilm.
These bacteria are responsible for chronic and serious infections in people with lung diseases, such as cystic fibrosis, and in chronic wounds. They also often cause pneumonia for people who are on ventilators and infections in burn victims.*
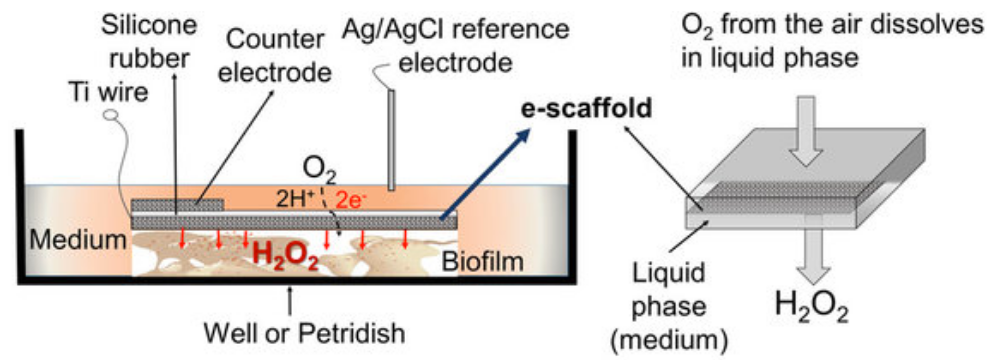
Schematic of experimental setup for the treatment of biofilm exposed to an e-scaffold, with an illustration of electrochemical production of hydrogen peroxide. The electrodes are connected to a potentiostat (600 mV source; not shown in figure). (credit: Sujala T. Sultana et al./Scientific Reports)
The researchers used an “e-scaffold” (electrochemical structure), a sort of electronic band-aid made out of conductive carbon fabric, which uses an electrical current to produce a low, constant concentration of hydrogen peroxide, an effective disinfectant, at the e-scaffold surface.
The hydrogen peroxide disrupts the biofilm (slime layer) matrix and damages the bacteria cell walls and DNA, which then allows better antibiotic penetration and efficacy against the bacteria. Bacteria that form biofilms are more difficult to kill because antibiotics only partially penetrate this protective layer. Subpopulations of “persister” cells survive treatment and are able to grow and multiply, resulting in chronic infections.
Tuning electrical stimulation to kill bacteria
Researchers have previously tried electrical stimulation, a method used to kill bacteria, for more than a century, but with limited results. In this study, the researchers determined the conditions necessary for the electrochemical reaction to produce hydrogen peroxide. The current has to be carefully controlled to assure the correct reaction at an exact rate to stop the bacteria from developing resistance, while not damaging surrounding tissue.**
The research to develop the e-scaffold actually came out of a failed attempt to improve fuel cells, according to research team leader Haluk Beyenal, the Paul Hohenschuh Distinguished Professor in WSU’s Gene and Linda Voiland School of Chemical Engineering and Bioengineering. When the researchers figured out they could only produce a small amount of electric current for their fuel cell cathode, they decided to see if they could use the process for a different purpose.
The technology may eventually be used to treat chronic wound infections, the researchers say. The researchers have filed a patent application and are working to commercialize the process. They hope to begin conducting clinical tests.
The work, reported in the online edition of npj Biofilms and Microbiomes, was supported by Beyenal’s National Science Foundation CAREER award.
* Bacterial resistance is a growing problem around the world. While antibiotics were a miracle drug of the 20th century, their widespread use has led to drug-resistant strains of bacteria. In the U.S., at least 2 million infections and 23,000 deaths are attributable to antibiotic-resistant bacteria each year, according to the U.S. Centers for Disease Control.
** As the researchers note in the paper, alternative antimicrobial treatments such as silver or mannitol, or in combination with conventional antibiotics, have toxic side effects at adequately high concentrations, and at low concentrations they often decompose before completely eliminating biofilm communities. In addition, persister cells can regrow and form biofilms with potentially enhanced tolerance to antibiotics.
Abstract of Eradication of Pseudomonas aeruginosa biofilms and persister cells using an electrochemical scaffold and enhanced antibiotic susceptibility
Biofilms in chronic wounds are known to contain a persister subpopulation that exhibits enhanced multidrug tolerance and can quickly rebound after therapeutic treatment. The presence of these “persister cells” is partly responsible for the failure of antibiotic therapies and incomplete elimination of biofilms. Electrochemical methods combined with antibiotics have been suggested as an effective alternative for biofilm and persister cell elimination, yet the mechanism of action for improved antibiotic efficacy remains unclear. In this work, an electrochemical scaffold (e-scaffold) that electrochemically generates a constant concentration of H2O2 was investigated as a means of enhancing tobramycin susceptibility in pre-grown Pseudomonas aeruginosa PAO1 biofilms and attacking persister cells. Results showed that the e-scaffold enhanced tobramycin susceptibility in P. aeruginosa PAO1 biofilms, which reached a maximum susceptibility at 40 µg/ml tobramycin, with complete elimination (7.8-log reduction vs control biofilm cells, P ≤ 0.001). Moreover, the e-scaffold eradicated persister cells in biofilms, leaving no viable cells (5-log reduction vs control persister cells, P ≤ 0.001). It was observed that the e-scaffold induced the intracellular formation of hydroxyl free radicals and improved membrane permeability in e-scaffold treated biofilm cells, which possibly enhanced antibiotic susceptibility and eradicated persister cells. These results demonstrate a promising advantage of the e-scaffold in the treatment of persistent biofilm infections.