Engineered ‘mini-organs’ produce insulin in mice
February 29, 2016
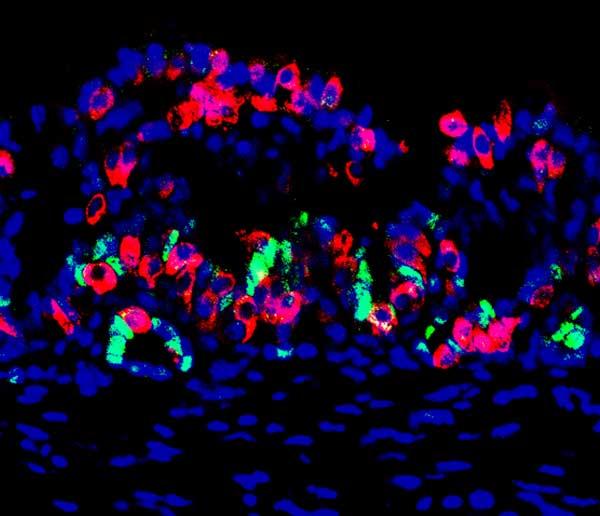
This mouse mini-organ contains stomach cells (red) that were reprogrammed to be able to produce insulin, along with some stem cells (green) that are able to replenish insulin-producing cells that are later destroyed after the mini-organ is transplanted into the mouse to supply insulin; the nuclei of all cells are shown in blue. (credit: Chaiyaboot Ariyachet)
Harvard University scientists have made major progress in dealing with a long-standing hurdle in treating diabetic patients.
People with diabetes have high blood sugar because their pancreatic beta cells (which store and release insulin) are not producing enough insulin. In type 1 diabetes (“juvenile diabetes”), the beta cells are even destroyed. In most cases, physicians treat type 1 diabetes with insulin injections, but people with complications may require pancreas implants or pancreatic islets from another person to replace those defective beta cells.
Those are limited. When effective sources of insulin-producing cells become available in the future, replacing beta cells with transplants would be preferable because that may enable blood sugar to be better controlled without the need for lifetime injections. The problem: those insulin-producing transplants tend to be destroyed by the ongoing diabetic disease process of the person (or animal) receiving the transplant.
Ideally, for a successful transplant to survive, transplanted cells or tissues need to somehow replenish or regenerate themselves.
Reprogramming stomach cells
In the new Harvard study, published online in an open access paper in Cell Stem Cell, the researchers have finally worked out a way to do that. They discovered that the lower stomach contains cells that can be reprogrammed to act as beta cells, and thus produce insulin. The stomach and intestine also naturally contain large numbers of stem cells that can multiply to continue to replenish the dying insulin-producing cells.
The report’s senior author, Qiao Zhou, PhD, associate professor at Harvard University’s Department of Stem Cell and Regenerative Biology, made the discovery after first genetically engineering mice to express three genes that can turn other cell types into insulin-producing beta-like cells. The researchers then looked for the tissue in the body that could be most easily reprogrammed to produce insulin.
“We looked all over, from the nose to the tail of the mouse,” says Zhou. “We discovered, surprisingly, that some of the cells in the pylorus region of the stomach (connecting the stomach to the small intestine) are most amenable to conversion to beta cells. This tissue appears to be the best starting material.”*
The team found that these stomach cells were naturally similar to pancreatic beta cells and could produce insulin and reverse high blood sugar in mice for up to six months.**
Creating “mini-stomachs” for humans containing insulin-producing cells for transplantation
Engineering a gastric mini-organ. An implanted bio-scafford induces beta-like cells (green) and gastric progenitor stem cells (blue). Blood vessels are shown in red. (credit: Chaiyaboot Ariyachet/Cell Stem Cell)
These initial experiments involved genetic engineering. But humans cannot yet be genetically engineered, so the researchers needed to find a version of this method that works for humans.
So the researchers took samples of the stomach tissue containing stem cells from the mice and grew them into “mini-organs” — tiny ball-like mini-stomachs that contain insulin-producing cells (that function like beta cells), as well as stem cells that can multiply to replace the insulin-producing cells as they later die after transplantation.
They transplanted these mini-organs into a membrane in the mouse’s abdomen. To test their function, the researchers then destroyed the normal insulin-producing pancreatic beta cells in the mice. They confirmed that the mini-organs compensated for the resulting loss of insulin, maintaining glucose at normal levels in five of the 22 experimental animals (this was the team’s expected success rate).
What is potentially really great about this approach (once it’s developed for humans) is that a physician will be able to create a patient-specific therapy by simply doing a biopsy (snipping out tissue for analysis) from the patient, grow the cells in the lab (as with the mice), reprogram those cells to produce insulin — and then transplant these cells directly into the patient.**
“That’s what we’re working on now,” says Zhou. “We’re very excited.”
Research funding was provided by the National Institute of Health, the HSCI, the Timothy Murphy Fund, the Children’s Hospital Boston Intellectual and Developmental Disabilities Research Center, and the Harvard Digestive Diseases Center.
* When engineered using reprogramming factors, cells in the pylorus region of the stomach were the most responsive to high glucose levels and produced insulin to normalize blood sugar. The researchers destroyed pancreatic beta cells in the mice, forcing their bodies to rely only on insulin produced by the reprogrammed stomach cells. Mice without tissue reprogramming died within eight weeks. But mice with reprogrammed cells maintained insulin and glucose levels in their blood for up to six months, which was as long as the experiment continued. The pyloric stomach also has the advantage of containing stem cells that, once reprogrammed, can replenish the insulin-producing cell population.
** These cells from the stomach are actually “beta-like cells” for producing insulin; that is, they do the same job as pancreatic beta cells. In the Cell Stem Cell paper, they are referred to as “insulin+ (insulin-positive)” cells.
Abstract of Reprogrammed Stomach Tissue as a Renewable Source of Functional β Cells for Blood Glucose Regulation
The gastrointestinal (GI) epithelium is a highly regenerative tissue with the potential to provide a renewable source of insulin+ cells after undergoing cellular reprogramming. Here, we show that cells of the antral stomach have a previously unappreciated propensity for conversion into functional insulin-secreting cells. Native antral endocrine cells share a surprising degree of transcriptional similarity with pancreatic β cells, and expression of β cell reprogramming factors in vivo converts antral cells efficiently into insulin+ cells with close molecular and functional similarity to β cells. Induced GI insulin+ cells can suppress hyperglycemia in a diabetic mouse model for at least 6 months and regenerate rapidly after ablation. Reprogramming of antral stomach cells assembled into bioengineered mini-organs in vitro yielded transplantable units that also suppressed hyperglycemia in diabetic mice, highlighting the potential for development of engineered stomach tissues as a renewable source of functional β cells for glycemic control.