Engineering ‘backup’ mitochondrial genes to restore power to cells
September 16, 2016
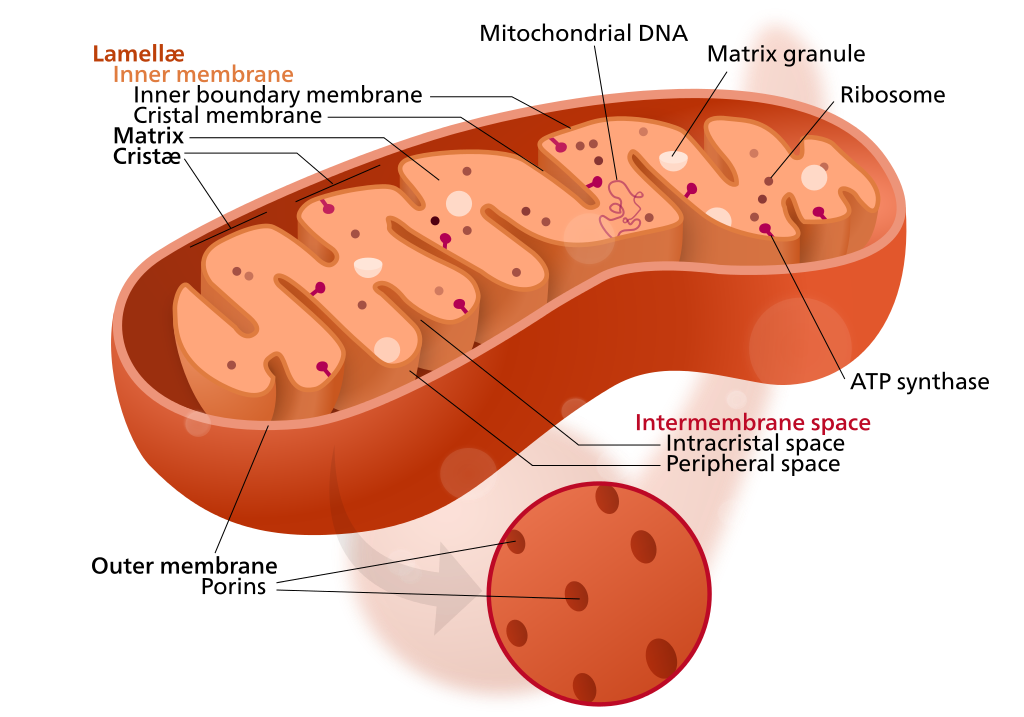
Mitochondrion structure (credit: Kelvinsong; modified by Sowlos/CC)
A new study by SENS Research Foundation, published in an open-access paper in the journal Nucleic Acids Research, explores the possibility of re-engineering mutated mitochondrial genes, which can otherwise lead to incurable disorders* and contribute to aging.
Mitochondria have their own DNA, allowing them to create proteins to supply nutrients and energy to cells. But sometimes, the DNA becomes mutated by “reactive oxygen species” generated by the mitochrondia themselves. This causes diseases in nervous, cardiovascular, and skeletal muscle tissues. More generally, “the mutations are also believed to contribute substantially to aging,” said Aubrey de Grey, PhD, a biomedical gerontologist and Chief Science Officer of SENS Research Foundation.
In the new study, the researchers developed “backup” copies of the modified mitochondrial genes, based on a patient cell line, to express the necessary proteins to produce cellular energy. The new genes would be delivered to the cell nucleii, where genes are protected from the more violent cell processes.**
The study was funded by the SENS Research Foundation, The Foster Foundation, Longecity Foundation, and Lifespan.io. NIH shared an instrumentation grant.
* One in 200 people are estimated to be born with a deleterious mitochondrial DNA mutation, leading to disorders such as Leber’s hereditary optic neuropathy, Leigh syndrome, mitochondrial encephalopathy, chronic progressive external ophthalmoplegia, and Kearns–Sayre syndrome, and also contribute to Parkinson’s.
** The researchers used a patient cell line with a single point mutation in the overlap region of the ATP8 and ATP6 genes of the human mitochondrial genome. Using synthesized DNA sequences, they were able to achieve stable expression, import, and function of the two mitochondria genes, correcting the loss of both mitochondrial proteins.
Singularity Lectures | Aubrey de Grey Announces Progress in MitoSENS
Abstract of Stable nuclear expression of ATP8 and ATP6 genes rescues a mtDNA Complex V null mutant
We explore the possibility of re-engineering mitochondrial genes and expressing them from the nucleus as an approach to rescue defects arising from mitochondrial DNA mutations. We have used a patient cybrid cell line with a single point mutation in the overlap region of the ATP8 andATP6 genes of the human mitochondrial genome. These cells are null for the ATP8 protein, have significantly lowered ATP6 protein levels and no Complex V function. Nuclear expression of only the ATP8 gene with theATP5G1 mitochondrial targeting sequence appended restored viability on Krebs cycle substrates and ATP synthesis capabilities but, failed to restore ATP hydrolysis and was insensitive to various inhibitors of oxidative phosphorylation. Co-expressing both ATP8 and ATP6 genes under similar conditions resulted in stable protein expression leading to successful integration into Complex V of the oxidative phosphorylation machinery. Tests for ATP hydrolysis / synthesis, oxygen consumption, glycolytic metabolism and viability all indicate a significant functional rescue of the mutant phenotype (including re-assembly of Complex V) following stable co-expression of ATP8 and ATP6. Thus, we report the stable allotopic expression, import and function of two mitochondria encoded genes,ATP8 and ATP6, resulting in simultaneous rescue of the loss of both mitochondrial proteins.