Engineering tough, resistant self-assembling amyloid fibers
January 29, 2015
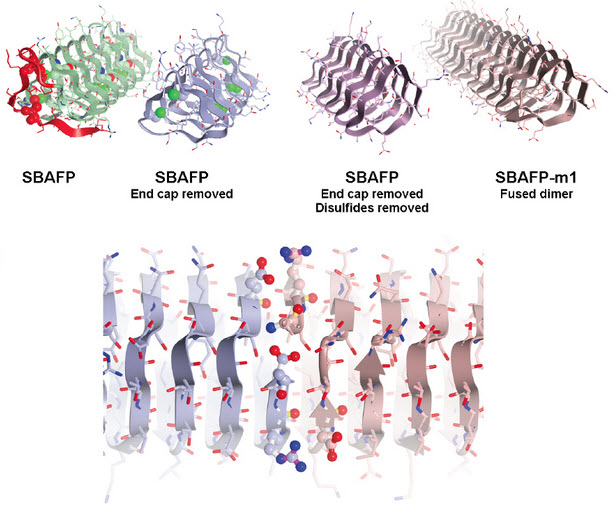
UC Davis researchers have engineered proteins so they spontaneously self-assemble into amyloid fibers. Here, the cap structure (red) was removed from spruce budworm antifreeze protein and other structures adjusted so that molecules could link up as fibrils (bottom). (Credit: UC Davis)
Researchers at UC Davis and Rice University have developed methods to manipulate natural proteins so that they self-assemble into amyloid fibrils.*
“These are big proteins with lots of flat surfaces suitable for functionalization, for example to grow photovoltaics or to attach to other surfaces,” said Dan Cox, a physics professor at UC Davis and coauthor on the paper. The fibers could also be used as “scaffolding” for tissue engineering, and potentially could be programmed so that other particles or proteins could be attached in specific locations or arrays.
Amyloids are also tough: they can withstand boiling, attack by digestive proteins, and ultraviolet radiation.
Maria Peralta, a graduate student in chemistry at UC Davis, and colleagues made the amyloid fibrils by tweaking natural “antifreeze” proteins from ryegrass and an insect, spruce budworm. These proteins allow some plants and animals to withstand very cold temperatures by preventing the growth of ice crystals, but they do not naturally self-assemble into larger structures.
To do that, the researchers removed cap structures from the end of the antifreeze proteins. The proteins could then self-assemble into fibrils with predictable heights — a potential new material for bioengineering.
The research paper was published online in the journal ACS Nano. The project was funded by the UC Davis Office of Research. Rice University scientists were also involved in the study.
*Amyloid proteins, for example, can self-assemble into the tangled plaques associated with Alzheimer’s disease — but similar proteins can also form very useful materials, such as spider silk, or biofilms around living cells.
Abstract of Engineering amyloid fibrils from β-solenoid proteins for biomaterials applications
Nature provides numerous examples of self-assembly that can potentially be implemented for materials applications. Considerable attention has been given to one-dimensional cross-β or amyloid structures that can serve as templates for wire growth or strengthen materials such as glue or cement. Here, we demonstrate controlled amyloid self-assembly based on modifications of β-solenoid proteins. They occur naturally in several contexts (e.g., antifreeze proteins, drug resistance proteins) but do not aggregate in vivo due to capping structures or distortions at their ends. Removal of these capping structures and regularization of the ends of the spruce budworm and rye grass antifreeze proteins yield micron length amyloid fibrils with predictable heights, which can be a platform for biomaterial-based self-assembly. The design process, including all-atom molecular dynamics simulations, purification, and self-assembly procedures are described. Fibril formation with the predicted characteristics is supported by evidence from thioflavin-T fluorescence, circular dichroism, dynamic light scattering, and atomic force microscopy. Additionally, we find evidence for lateral assembly of the modified spruce budworm antifreeze fibrils with sufficient incubation time. The kinetics of polymerization are consistent with those for other amyloid formation reactions and are relatively fast due to the preformed nature of the polymerization nucleus.