First working synthetic immune organ with controllable antibodies
June 11, 2015
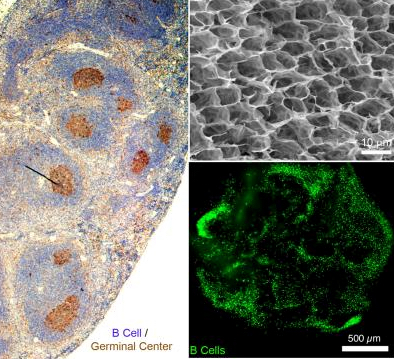
When exposed to a foreign agent, such as an immunogenic protein, B cells in lymphoid organs (such as the spleen) undergo germinal center (immune defense) reactions. The image on the left is an normal immunized mouse spleen with activated B cells (brown) that produce antibodies. At right, top: a scanning electron micrograph of synthetic porous synthetic immune organoids that enable rapid proliferation and activation of B cells into antibody-producing cells. At right, bottom: primary B cell viability and distribution is visible 24 hours following encapsulation of B cells from the mouse lymphoid organ into the synthetic organoids. (credit: Singh Lab)
Cornell University engineers have created a functional, synthetic immune organoid (a lab-grown ball of cells with some of the features of a normal organ) that produces antibodies. The engineered organ has implications for everything from rapid production of immune therapies to new frontiers in cancer or infectious disease research.
The first-of-its-kind immune organoid was created in the lab of Ankur Singh, assistant professor of mechanical and aerospace engineering, who applies engineering principles to the study and manipulation of the human immune system.
Simulating the body’s immune response
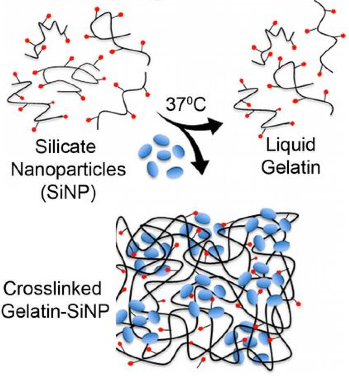
Silicate nanoparticles (SiNP) are used for ionic crosslinking of gelatin to form stable hydrogel at body temperature (credit: Alberto Purwada et al./Biomaterials)
The synthetic organ is bio-inspired by secondary immune organs like the lymph node or spleen. It is made from a hydrogel (a soft, nanocomposite gelatin-like biomaterial), reinforced with silicate nanoparticles to keep the structure from melting at body temperature.
This biomaterial is also seeded with B cells. It mimics the body’s normal anatomical microenvironment of lymphoid tissue, which produces lymphocytes and antibodies in the lymph nodes, thymus, tonsils, and spleen.
Like a real organ, the organoid converts B cells — which make antibodies that respond to infectious invaders — into germinal centers, which are clusters of B cells that activate, mature and mutate their antibody genes when the body is under attack. (Germinal centers are a sign of infection and are not present in healthy immune organs.)
The engineers have demonstrated how they can control this immune response in the organ and tune how quickly the B cells proliferate, get activated and change their antibody types. According to their paper, their 3-D organ outperforms existing 2-D lab cultures and can produce activated B cells up to 100 times faster.
A new tool for studying immune functions
According to Singh, the organoid could lead to increased understanding of B cell functions, an area of study that typically relies on animal models to observe how the cells develop and mature, and could also be used to study specific infections and how the body produces antibodies to fight those infections — from Ebola to HIV.
“You can use our system to force the production of immunotherapeutics at much faster rates,” he said. Such a system also could be used to test toxic chemicals and environmental factors that contribute to infections or organ malfunctions.
The process of B cells becoming germinal centers is not well understood, and in fact, when the body makes mistakes in the genetic rearrangement related to this process, blood cancer can result.
“In the long run, we anticipate that the ability to drive immune reaction ex vivo [outside the body] at controllable rates grants us the ability to reproduce immunological events with tunable parameters for better mechanistic understanding of B cell development and generation of B cell tumors, as well as screening and translation of new classes of drugs,” Singh said.
The work was published online June 3 in Biomaterials and will appear later in print.
Abstract of Ex vivo Engineered Immune Organoids for Controlled Germinal Center Reactions
Ex vivo engineered three-dimensional organotypic cultures have enabled the real-time study and control of biological functioning of mammalian tissues. Organs of broad interest where its architectural, cellular, and molecular complexity has prevented progress in ex vivo engineering are the secondary immune organs. Ex vivo immune organs can enable mechanistic understanding of the immune system and more importantly, accelerate the translation of immunotherapies as well as a deeper understanding of the mechanisms that lead to their malignant transformation into a variety of B and T cell malignancies. However, till date, no modular ex vivo immune organ has been developed with an ability to control the rate of immune reaction through tunable design parameter. Here we describe a B cell follicle organoid made of nanocomposite biomaterials, which recapitulates the anatomical microenvironment of a lymphoid tissue that provides the basis to induce an accelerated germinal center (GC) reaction by continuously providing extracellular matrix (ECM) and cell-cell signals to naïve B cells. Compared to existing co-cultures, immune organoids provide a control over primary B cell proliferation with ∼100-fold higher and rapid differentiation to the GC phenotype with robust antibody class switching.